Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved Efficacy and Reduced Toxicity Using a Custom-Designed Irinotecan-Delivering Silicasome for Orthotopic Colon Cancer.
ACS Nano ( IF 15.8 ) Pub Date : 2018-12-11 , DOI: 10.1021/acsnano.8b06164 Xiangsheng Liu 1, 2 , Jinhong Jiang 2 , Ryan Chan 1 , Ying Ji 1 , Jianqin Lu 1, 2 , Yu-Pei Liao 1 , Michael Okene 1 , Joshua Lin 1 , Paulina Lin 1 , Chong Hyun Chang 2 , Xiang Wang 2 , Ivanna Tang 1 , Emily Zheng 1 , Waveley Qiu 1 , Zev A Wainberg 3 , Andre E Nel 1, 2, 4 , Huan Meng 1, 2, 4
ACS Nano ( IF 15.8 ) Pub Date : 2018-12-11 , DOI: 10.1021/acsnano.8b06164 Xiangsheng Liu 1, 2 , Jinhong Jiang 2 , Ryan Chan 1 , Ying Ji 1 , Jianqin Lu 1, 2 , Yu-Pei Liao 1 , Michael Okene 1 , Joshua Lin 1 , Paulina Lin 1 , Chong Hyun Chang 2 , Xiang Wang 2 , Ivanna Tang 1 , Emily Zheng 1 , Waveley Qiu 1 , Zev A Wainberg 3 , Andre E Nel 1, 2, 4 , Huan Meng 1, 2, 4
Affiliation
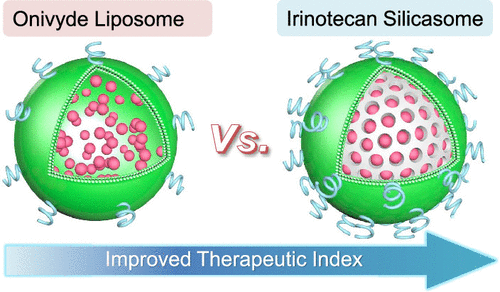
|
Irinotecan is a key chemotherapeutic agent for the treatment of colorectal (CRC) and pancreatic (PDAC) cancer. Because of a high incidence of bone marrow and gastrointestinal (GI) toxicity, Onivyde (a liposome) was introduced to provide encapsulated irinotecan (Ir) delivery in PDAC patients. While there is an ongoing clinical trial (NCT02551991) to investigate the use of Onivyde as a first-line option to replace irinotecan in FOLFIRINOX, the liposomal formulation is currently prescribed as a second-line treatment option (in combination with 5-fluorouracil and leucovorin) for patients with metastatic PDAC who failed gemcitabine therapy. However, the toxicity of Onivyde remains a concern that needs to be addressed for use in CRC as well. Our goal was to custom design a mesoporous silica nanoparticle (MSNP) carrier for encapsulated irinotecan delivery in a robust CRC model. This was achieved by developing an orthotopic tumor chunk model in immunocompetent mice. With a view to increase the production volume and to expand the disease applications, the carrier design was improved by using an ethanol exchange method for coating of a supported lipid bilayer (LB) that entraps a protonating agent. The encapsulated protonating agent was subsequently used for remote loading of irinotecan. The excellent irinotecan loading capacity and stability of the LB-coated MSNP carrier, also known as a "silicasome", previously showed improved efficacy and reduced toxicity when compared to an in-house liposomal carrier in a PDAC model. Intravenous injection of the silicasomes in a well-developed orthotopic colon cancer model in mice demonstrated improved pharmacokinetics and tumor drug content over free drug and Onivyde. Moreover, improved drug delivery was accompanied by substantially improved efficacy, increased survival, and reduced bone marrow and GI toxicity compared to the free drug and Onivyde. We also confirmed that the custom-designed irinotecan silicasomes outperform Onivyde in an orthotopic PDAC model. In summary, the Ir-silicasome appears to be promising as a treatment option for CRC in humans based on improved efficacy and the carrier's favorable safety profile.
中文翻译:

使用定制设计的伊立替康递送二氧化硅小体治疗原位结肠癌可提高疗效并降低毒性。
伊立替康是用于治疗结直肠癌(CRC)和胰腺癌(PDAC)的关键化学治疗剂。由于骨髓和胃肠道(GI)毒性的发生率很高,因此引入了Onivyde(脂质体)为PDAC患者提供胶囊化的伊立替康(Ir)递送。虽然正在进行一项临床试验(NCT02551991),以研究使用Onivyde作为一线选择替代FOLFIRINOX中的伊立替康,但目前已将脂质体制剂作为第二线治疗选择(与5-氟尿嘧啶和亚叶酸钙结合使用) )用于吉西他滨治疗失败的转移性PDAC患者。然而,Onivyde的毒性仍然是一个需要关注的问题,也需要在CRC中使用。我们的目标是定制设计介孔二氧化硅纳米颗粒(MSNP)载体,用于在稳健的CRC模型中封装伊立替康。这是通过在免疫功能正常的小鼠体内开发原位肿瘤块模型来实现的。为了增加生产量并扩大疾病的应用范围,通过使用乙醇交换方法涂覆包封质子化剂的负载脂质双层(LB),改进了载体设计。随后将包封的质子化剂用于伊立替康的远程装载。与PDAC模型中的内部脂质体载体相比,LB包被的MSNP载体(也称为“二氧化硅小体”)具有出色的伊立替康负载能力和稳定性,以前显示出更高的功效和更低的毒性。在成熟的原位结肠癌小鼠模型中静脉内注射二氧化硅脂质体,与游离药物和Onivyde相比,显示出更高的药代动力学和肿瘤药物含量。此外,与游离药物和Onivyde相比,改善的药物递送具有显着改善的功效,增加的生存率以及降低的骨髓和胃肠道毒性。我们还证实,在原位PDAC模型中,定制设计的伊立替康二氧化硅脂质体优于Onivyde。总而言之,基于改善的疗效和载体的良好安全性,Ir-二氧化硅小体似乎有望成为人类CRC的治疗选择。与游离药物和Onivyde相比,药物递送的改善伴随着疗效的显着提高,存活率的提高以及骨髓和胃肠道毒性的降低。我们还证实,在原位PDAC模型中,定制设计的伊立替康二氧化硅脂质体优于Onivyde。总而言之,基于改善的疗效和载体的良好安全性,Ir-二氧化硅小颗粒似乎有望成为人类CRC的治疗选择。与游离药物和Onivyde相比,药物递送的改善伴随着疗效的显着提高,存活率的提高以及骨髓和胃肠道毒性的降低。我们还证实,在原位PDAC模型中,定制设计的伊立替康二氧化硅脂质体优于Onivyde。总而言之,基于改善的疗效和载体的良好安全性,Ir-二氧化硅小体似乎有望成为人类CRC的治疗选择。
更新日期:2018-12-11
中文翻译:

使用定制设计的伊立替康递送二氧化硅小体治疗原位结肠癌可提高疗效并降低毒性。
伊立替康是用于治疗结直肠癌(CRC)和胰腺癌(PDAC)的关键化学治疗剂。由于骨髓和胃肠道(GI)毒性的发生率很高,因此引入了Onivyde(脂质体)为PDAC患者提供胶囊化的伊立替康(Ir)递送。虽然正在进行一项临床试验(NCT02551991),以研究使用Onivyde作为一线选择替代FOLFIRINOX中的伊立替康,但目前已将脂质体制剂作为第二线治疗选择(与5-氟尿嘧啶和亚叶酸钙结合使用) )用于吉西他滨治疗失败的转移性PDAC患者。然而,Onivyde的毒性仍然是一个需要关注的问题,也需要在CRC中使用。我们的目标是定制设计介孔二氧化硅纳米颗粒(MSNP)载体,用于在稳健的CRC模型中封装伊立替康。这是通过在免疫功能正常的小鼠体内开发原位肿瘤块模型来实现的。为了增加生产量并扩大疾病的应用范围,通过使用乙醇交换方法涂覆包封质子化剂的负载脂质双层(LB),改进了载体设计。随后将包封的质子化剂用于伊立替康的远程装载。与PDAC模型中的内部脂质体载体相比,LB包被的MSNP载体(也称为“二氧化硅小体”)具有出色的伊立替康负载能力和稳定性,以前显示出更高的功效和更低的毒性。在成熟的原位结肠癌小鼠模型中静脉内注射二氧化硅脂质体,与游离药物和Onivyde相比,显示出更高的药代动力学和肿瘤药物含量。此外,与游离药物和Onivyde相比,改善的药物递送具有显着改善的功效,增加的生存率以及降低的骨髓和胃肠道毒性。我们还证实,在原位PDAC模型中,定制设计的伊立替康二氧化硅脂质体优于Onivyde。总而言之,基于改善的疗效和载体的良好安全性,Ir-二氧化硅小体似乎有望成为人类CRC的治疗选择。与游离药物和Onivyde相比,药物递送的改善伴随着疗效的显着提高,存活率的提高以及骨髓和胃肠道毒性的降低。我们还证实,在原位PDAC模型中,定制设计的伊立替康二氧化硅脂质体优于Onivyde。总而言之,基于改善的疗效和载体的良好安全性,Ir-二氧化硅小颗粒似乎有望成为人类CRC的治疗选择。与游离药物和Onivyde相比,药物递送的改善伴随着疗效的显着提高,存活率的提高以及骨髓和胃肠道毒性的降低。我们还证实,在原位PDAC模型中,定制设计的伊立替康二氧化硅脂质体优于Onivyde。总而言之,基于改善的疗效和载体的良好安全性,Ir-二氧化硅小体似乎有望成为人类CRC的治疗选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号