当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Properties of Benzo‐Fused Indeno[2,1‐c]fluorenes
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2019-01-07 , DOI: 10.1002/asia.201801684 Tanguy Jousselin‐Oba 1 , Parker E. Deal 2 , Aaron G. Fix 2 , Conerd K. Frederickson 2 , Chris L. Vonnegut 2 , Abderrahim Yassar 3 , Lev N. Zakharov 4 , Michel Frigoli 1 , Michael M. Haley 2
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2019-01-07 , DOI: 10.1002/asia.201801684 Tanguy Jousselin‐Oba 1 , Parker E. Deal 2 , Aaron G. Fix 2 , Conerd K. Frederickson 2 , Chris L. Vonnegut 2 , Abderrahim Yassar 3 , Lev N. Zakharov 4 , Michel Frigoli 1 , Michael M. Haley 2
Affiliation
|
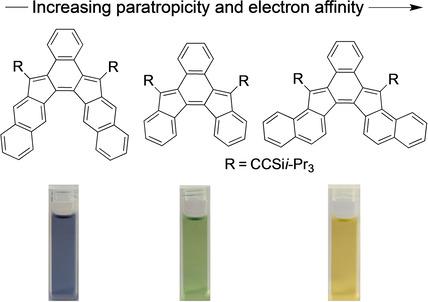
A set of fully‐conjugated indenofluorenes has been synthesized and confirmed by solid‐state structure analysis. The indeno[2,1‐c]fluorenes and their benzo‐fused analogues all contain the antiaromatic as‐indacene core. The molecules possess high electron affinities and show a broad absorption that reaches into the near‐IR region of the electromagnetic spectrum. All of the featured compounds reversibly accept up to two electrons as revealed by cyclic voltammetry. Analysis of molecule tropicity using NICS‐XY scan calculations shows that, while the as‐indacene core is less paratropic than s‐indacene, benz[a]‐annulation further reduces the antiaromaticity of the core. Antiaromatic strength of the as‐indacene core can also be tuned by the position of fusion of additional arenes on the outer rings.
中文翻译:

苯并茚并[2,1-c]芴的合成与性质
合成了一组完全共轭的茚并芴,并通过固态结构分析进行了确认。茚并[ 2,1– c ]芴及其苯并稠合类似物均含有抗芳烃作为茚满并烯的核心。这些分子具有很高的电子亲和力,并显示出广泛的吸收能力,可吸收到电磁光谱的近红外区域。如循环伏安法所揭示的,所有具有特征的化合物最多可逆地接受两个电子。使用NICS-XY扫描计算对分子的亲和性进行分析表明,尽管as-茚并二烯核的顺性性低于s-茚并二烯,但苯并[a]环化进一步降低了核的抗芳香性。的反芳香强度为还可以通过外圈上其他芳烃的融合位置来调节ind-茚并茂的核心。
更新日期:2019-01-07
中文翻译:

苯并茚并[2,1-c]芴的合成与性质
合成了一组完全共轭的茚并芴,并通过固态结构分析进行了确认。茚并[ 2,1– c ]芴及其苯并稠合类似物均含有抗芳烃作为茚满并烯的核心。这些分子具有很高的电子亲和力,并显示出广泛的吸收能力,可吸收到电磁光谱的近红外区域。如循环伏安法所揭示的,所有具有特征的化合物最多可逆地接受两个电子。使用NICS-XY扫描计算对分子的亲和性进行分析表明,尽管as-茚并二烯核的顺性性低于s-茚并二烯,但苯并[a]环化进一步降低了核的抗芳香性。的反芳香强度为还可以通过外圈上其他芳烃的融合位置来调节ind-茚并茂的核心。











































 京公网安备 11010802027423号
京公网安备 11010802027423号