当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modeling genome-wide enzyme evolution predicts strong epistasis underlying catalytic turnover rates.
Nature Communications ( IF 14.7 ) Pub Date : 2018-12-10 , DOI: 10.1038/s41467-018-07649-1 David Heckmann , Daniel C. Zielinski , Bernhard O. Palsson
Nature Communications ( IF 14.7 ) Pub Date : 2018-12-10 , DOI: 10.1038/s41467-018-07649-1 David Heckmann , Daniel C. Zielinski , Bernhard O. Palsson
|
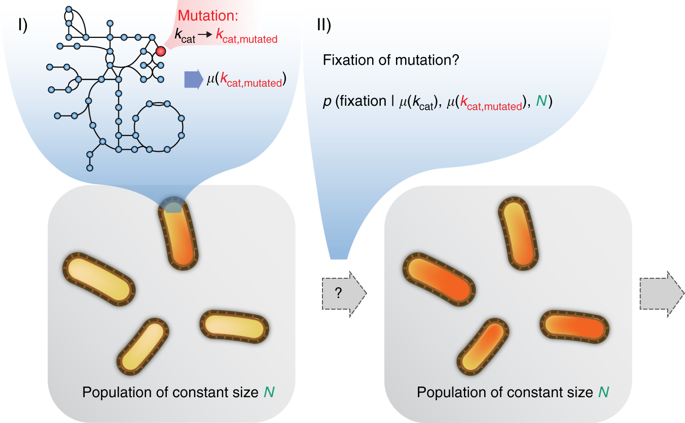
Systems biology describes cellular phenotypes as properties that emerge from the complex interactions of individual system components. Little is known about how these interactions have affected the evolution of metabolic enzymes. Here, we combine genome-scale metabolic modeling with population genetics models to simulate the evolution of enzyme turnover numbers (kcats) from a theoretical ancestor with inefficient enzymes. This systems view of biochemical evolution reveals strong epistatic interactions between metabolic genes that shape evolutionary trajectories and influence the magnitude of evolved kcats. Diminishing returns epistasis prevents enzymes from developing higher kcats in all reactions and keeps the organism far from the potential fitness optimum. Multifunctional enzymes cause synergistic epistasis that slows down adaptation. The resulting fitness landscape allows kcat evolution to be convergent. Predicted kcat parameters show a significant correlation with experimental data, validating our modeling approach. Our analysis reveals how evolutionary forces shape modern kcats and the whole of metabolism.
中文翻译:

对全基因组酶进化进行建模可预测潜在的催化转化率强上位性。
系统生物学将细胞表型描述为从单个系统组件的复杂相互作用中产生的特性。关于这些相互作用如何影响代谢酶的进化知之甚少。在这里,我们将基因组规模的代谢建模与种群遗传学模型相结合,以模拟从理论祖先到低效酶的酶转换数(k cat s)的演变。该系统关于生化进化的观点揭示了代谢基因之间强大的上位相互作用,这些基因塑造了进化轨迹并影响了进化的k cat s的大小。收益递减递减会阻止酶发展成更高的k cat会影响所有反应,并使有机体远离潜在的最佳适应性。多功能酶引起协同上位,从而减慢适应。由此产生的适应度景观使k cat进化收敛。预测的k cat参数显示出与实验数据的显着相关性,从而验证了我们的建模方法。我们的分析揭示了进化力如何塑造现代猫和整个新陈代谢。
更新日期:2018-12-10
中文翻译:

对全基因组酶进化进行建模可预测潜在的催化转化率强上位性。
系统生物学将细胞表型描述为从单个系统组件的复杂相互作用中产生的特性。关于这些相互作用如何影响代谢酶的进化知之甚少。在这里,我们将基因组规模的代谢建模与种群遗传学模型相结合,以模拟从理论祖先到低效酶的酶转换数(k cat s)的演变。该系统关于生化进化的观点揭示了代谢基因之间强大的上位相互作用,这些基因塑造了进化轨迹并影响了进化的k cat s的大小。收益递减递减会阻止酶发展成更高的k cat会影响所有反应,并使有机体远离潜在的最佳适应性。多功能酶引起协同上位,从而减慢适应。由此产生的适应度景观使k cat进化收敛。预测的k cat参数显示出与实验数据的显着相关性,从而验证了我们的建模方法。我们的分析揭示了进化力如何塑造现代猫和整个新陈代谢。











































 京公网安备 11010802027423号
京公网安备 11010802027423号