当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesizing Stilbene by Olefin Metathesis Reaction Using Guided Inquiry To Compare and Contrast Wittig and Metathesis Methodologies
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-12-06 00:00:00 , DOI: 10.1021/acs.jchemed.8b00313 Timothy J. Bannin 1 , Partha P. Datta 1 , Elizabeth T. Kiesewetter 2 , Matthew K. Kiesewetter 1
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-12-06 00:00:00 , DOI: 10.1021/acs.jchemed.8b00313 Timothy J. Bannin 1 , Partha P. Datta 1 , Elizabeth T. Kiesewetter 2 , Matthew K. Kiesewetter 1
Affiliation
|
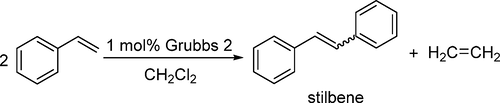
In this experiment, students are asked to conduct a catalytic cross-metathesis experiment and compare this reaction to the Wittig reaction within the confines of green chemistry. Students synthesize stilbene from styrene using Grubbs second-generation catalyst. Products can be minimally characterized by IR spectroscopy and melting point, but using 1H NMR spectroscopy is preferred. Students find that the Wittig reaction is selective for cis-stilbene while the metathesis reaction produces >98% trans-stilbene. Students determine the cis/trans selectivity, turnover number, and maximum turnover frequency of the reaction. The experiment is conducted alongside the synthesis of stilbene using Wittig chemistry from a published procedure.
中文翻译:

烯烃复分解反应合成的二苯乙烯的指导研究比较和对比Wittig和复分解方法
在该实验中,要求学生进行催化的交叉复分解实验,并将该反应与绿色化学范围内的Wittig反应进行比较。学生使用Grubbs第二代催化剂从苯乙烯合成二苯乙烯。可以通过红外光谱和熔点来最小限度地表征产物,但是优选使用1 H NMR光谱。学生发现,Wittig反应对顺式-lb具有选择性,而复分解反应可产生> 98%的反式-sti。学生确定顺式/反式反应的选择性,周转次数和最大周转频率。该实验与使用已公开程序中的Wittig化学方法合成二苯乙烯一起进行。
更新日期:2018-12-06
中文翻译:

烯烃复分解反应合成的二苯乙烯的指导研究比较和对比Wittig和复分解方法
在该实验中,要求学生进行催化的交叉复分解实验,并将该反应与绿色化学范围内的Wittig反应进行比较。学生使用Grubbs第二代催化剂从苯乙烯合成二苯乙烯。可以通过红外光谱和熔点来最小限度地表征产物,但是优选使用1 H NMR光谱。学生发现,Wittig反应对顺式-lb具有选择性,而复分解反应可产生> 98%的反式-sti。学生确定顺式/反式反应的选择性,周转次数和最大周转频率。该实验与使用已公开程序中的Wittig化学方法合成二苯乙烯一起进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号