当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Size‐Expanded Nucleobase Analogues for Artificial Base‐Pairing Using a Ligand‐Free, Microwave‐Assisted Copper(I)‐Catalyzed Reaction
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-12-07 , DOI: 10.1002/slct.201802455 K Radhakrishnan 1 , Soumi Das 1 , Lal Mohan Kundu 1
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-12-07 , DOI: 10.1002/slct.201802455 K Radhakrishnan 1 , Soumi Das 1 , Lal Mohan Kundu 1
Affiliation
|
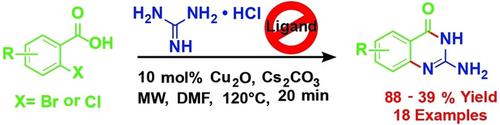
Abstract: Quinazolines is an important class of natural products. Size‐expanded isocytosine analogues or 2‐aminoquinazolinones have found significance as pharmaceutically active compounds, for the expansion of genetic alphabets and as biomolecular probes. With an aim for further biomolecular applications, a library of functionalized isocytosine analogues have been synthesized in a one‐pot, microwave‐directed reaction. Syntheses were carried out using simple reactants such as ortho‐halo aromatic carboxylic acids and guanidine hydrochloride, in absence of any additional ligand. The usefulness of the methodology could be justified by the diversity of the compounds synthesized, containing functional groups, natural nucleobases, fluorophores or peptide bonds.
中文翻译:

使用无配体的微波辅助铜(I)催化反应合成用于人工碱基配对的扩容核碱基类似物
摘要:喹唑啉是一类重要的天然产物。已发现大小扩展的异胞嘧啶类似物或2-氨基喹唑啉酮作为药物活性化合物,用于扩展遗传字母和作为生物分子探针具有重要意义。为了进一步的生物分子应用,已经在单锅微波定向反应中合成了功能化异胞嘧啶类似物的文库。使用简单的反应物(例如邻卤代芳族羧酸和盐酸胍)进行合成,没有任何其他配体。该方法的实用性可以通过合成的化合物的多样性来证明,这些化合物包含官能团,天然核碱基,荧光团或肽键。
更新日期:2018-12-07
中文翻译:

使用无配体的微波辅助铜(I)催化反应合成用于人工碱基配对的扩容核碱基类似物
摘要:喹唑啉是一类重要的天然产物。已发现大小扩展的异胞嘧啶类似物或2-氨基喹唑啉酮作为药物活性化合物,用于扩展遗传字母和作为生物分子探针具有重要意义。为了进一步的生物分子应用,已经在单锅微波定向反应中合成了功能化异胞嘧啶类似物的文库。使用简单的反应物(例如邻卤代芳族羧酸和盐酸胍)进行合成,没有任何其他配体。该方法的实用性可以通过合成的化合物的多样性来证明,这些化合物包含官能团,天然核碱基,荧光团或肽键。



























 京公网安备 11010802027423号
京公网安备 11010802027423号