当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Immobilized tetrakis(triphenylphosphine)palladium(0) for Suzuki–Miyaura coupling reactions under flow conditions†
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-12-03 00:00:00 , DOI: 10.1039/c8re00235e G. Valerie Ramaotsoa 1, 2, 3, 4, 5 , Ian Strydom 1, 2, 3, 4 , Jenny-Lee Panayides 4, 5 , Darren Riley 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-12-03 00:00:00 , DOI: 10.1039/c8re00235e G. Valerie Ramaotsoa 1, 2, 3, 4, 5 , Ian Strydom 1, 2, 3, 4 , Jenny-Lee Panayides 4, 5 , Darren Riley 1, 2, 3, 4
Affiliation
|
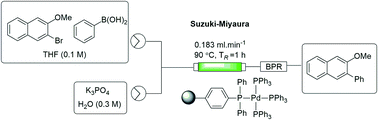
An immobilized triphenylphosphine scaffold was prepared by precipitation polymerization and functionalized to afford a cost-effective source of solid-supported tetrakis(triphenylphosphine)palladium(0). The catalyst was characterised and used to perform biphasic Suzuki–Miyaura cross-coupling reactions using a packed-bed reactor under flow conditions. The approach afforded comparable yields to those obtained under batch conditions with a single pass through the packed-bed reactor (1 h vs. 18 h). The use of a recycling system was investigated on a model reaction and found to afford close to quantitative conversion within 3 hours.
中文翻译:

流动条件下用于铃木-宫浦偶联反应的固定化四(三苯基膦)钯(0)†
通过沉淀聚合制备固定化的三苯基膦支架并进行功能化,以提供经济高效的固体负载四(三苯基膦)钯(0)来源。对该催化剂进行了表征,并在流动条件下使用填充床反应器进行了两相Suzuki-Miyaura交叉偶联反应。该方法提供的收率与在单次通过填充床反应器的分批条件下获得的收率相当(1小时vs. 18小时)。在模型反应中研究了循环系统的使用,发现在3小时内可达到接近定量的转化率。
更新日期:2018-12-03
中文翻译:

流动条件下用于铃木-宫浦偶联反应的固定化四(三苯基膦)钯(0)†
通过沉淀聚合制备固定化的三苯基膦支架并进行功能化,以提供经济高效的固体负载四(三苯基膦)钯(0)来源。对该催化剂进行了表征,并在流动条件下使用填充床反应器进行了两相Suzuki-Miyaura交叉偶联反应。该方法提供的收率与在单次通过填充床反应器的分批条件下获得的收率相当(1小时vs. 18小时)。在模型反应中研究了循环系统的使用,发现在3小时内可达到接近定量的转化率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号