当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Criegee Intermediate Reaction with Alcohol Is Enhanced by a Single Water Molecule
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-12-04 00:00:00 , DOI: 10.1021/acs.jpclett.8b03349 Yen-Hsiu Lin,Cangtao Yin,Wei-Hong Lin,Yu-Lin Li,Kaito Takahashi,Jim Jr-Min Lin
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-12-04 00:00:00 , DOI: 10.1021/acs.jpclett.8b03349 Yen-Hsiu Lin,Cangtao Yin,Wei-Hong Lin,Yu-Lin Li,Kaito Takahashi,Jim Jr-Min Lin
|
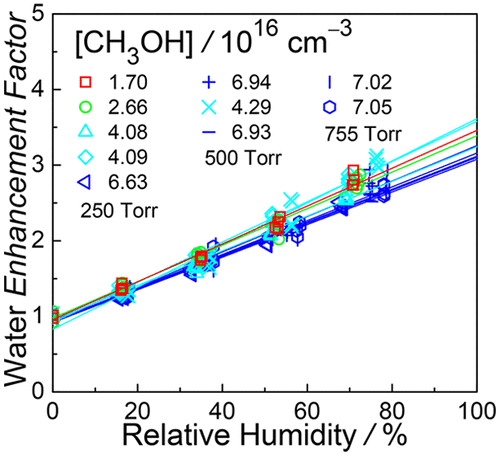
The role of water in gas-phase reactions has gained considerable interest. Here we report a direct kinetic measurement of the reaction of syn-CH3CHOO (a Criegee intermediate or carbonyl oxide) with methanol at various relative humidity (RH = 0–80%) under near-ambient conditions (298 K, 250–755 Torr). The data indicate that a single water molecule expedites the reaction by up to a factor of three. The rate coefficient of the corresponding reaction, syn-CH3CHOO + CH3OH + H2O → products, has been determined to be (1.95 ± 0.11) × 10–32 cm6 s–1 at 298 K, with no observable pressure dependence for 250–755 Torr. Quantum chemistry calculation shows that the dominating pathway involves a hydrogen-bonded ring structure, in which methanol is donating a hydrogen atom to water, water is donating a hydrogen atom to the terminal oxygen atom of the Criegee intermediate, and, on the product side, H2O is reformed and acts as a catalyst.
中文翻译:

单一水分子增强了Criegee与酒精的中间反应
水在气相反应中的作用已引起人们极大的兴趣。在这里,我们报告了在接近室温(298 K,250-755)的各种相对湿度(RH = 0–80%)下,syn -CH 3 CHOO(一种Criegee中间体或羰基氧化物)与甲醇反应的直接动力学测量。r)。数据表明单个水分子最多可将反应速度提高三倍。相应反应的速率系数syn -CH 3 CHOO + CH 3 OH + H 2 O→产物已确定为(1.95±0.11)×10 –32 cm 6 s –1在298 K时,对于250–755 Torr没有明显的压力依赖性。量子化学计算表明,主要途径涉及氢键合的环结构,其中甲醇将氢原子提供给水,水将氢原子提供给Criegee中间体的末端氧原子,在产物侧, H 2 O被重整并充当催化剂。
更新日期:2018-12-04
中文翻译:

单一水分子增强了Criegee与酒精的中间反应
水在气相反应中的作用已引起人们极大的兴趣。在这里,我们报告了在接近室温(298 K,250-755)的各种相对湿度(RH = 0–80%)下,syn -CH 3 CHOO(一种Criegee中间体或羰基氧化物)与甲醇反应的直接动力学测量。r)。数据表明单个水分子最多可将反应速度提高三倍。相应反应的速率系数syn -CH 3 CHOO + CH 3 OH + H 2 O→产物已确定为(1.95±0.11)×10 –32 cm 6 s –1在298 K时,对于250–755 Torr没有明显的压力依赖性。量子化学计算表明,主要途径涉及氢键合的环结构,其中甲醇将氢原子提供给水,水将氢原子提供给Criegee中间体的末端氧原子,在产物侧, H 2 O被重整并充当催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号