当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the cooperativity between multiple interactions of dimethyl sulfoxide with carbon dioxide and water
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2018-12-03 , DOI: 10.1002/jcc.25732 Pham N. Khanh 1 , Cam‐Tu D. Phan 1 , Dai Q. Ho 1 , Quan Van Vo 2 , Vu T. Ngan 1 , Minh Tho Nguyen 3 , Nguyen T. Trung 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2018-12-03 , DOI: 10.1002/jcc.25732 Pham N. Khanh 1 , Cam‐Tu D. Phan 1 , Dai Q. Ho 1 , Quan Van Vo 2 , Vu T. Ngan 1 , Minh Tho Nguyen 3 , Nguyen T. Trung 1
Affiliation
|
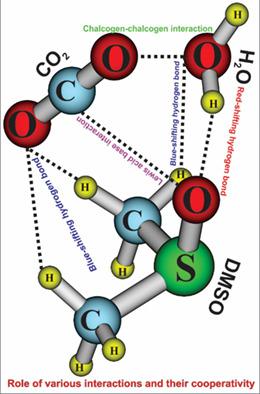
Interactions of dimethyl sulfoxide with carbon dioxide and water molecules which induce 18 significantly stable complexes are thoroughly investigated. An addition of CO2 or H2O molecules into the DMSO⋯1CO2 and DMSO⋯1H2O systems leads to an increase in the stability of the resulting complexes, in which it is larger for a H2O addition than a CO2. The overall stabilization energy of the DMSO⋯1,2CO2 is mainly contributed by the S=O⋯C Lewis acid–base interaction, whereas the O − H⋯O hydrogen bond plays a significant role in stabilizing complexes of DMSO⋯1,2H2O and DMSO⋯1CO2⋯1H2O. Remarkably, the complexes of DMSO⋯2H2O are found to be more stable than DMSO⋯1CO2⋯1H2O and DMSO⋯2CO2. The level of the cooperativity of multiple interactions in ternary complexes tends to decrease in going from DMSO⋯2H2O to DMSO⋯1CO2⋯1H2O and finally to DMSO⋯2CO2. It is generally found that the red shift of the O − H bond involved in an O − H⋯O hydrogen bond increases while the blue shift of a C − H bond in a C − H⋯O hydrogen bond decreases when a cooperative effect occurs in ternary complexes as compared to those of the corresponding binary complexes. © 2018 Wiley Periodicals, Inc.
中文翻译:

深入了解二甲基亚砜与二氧化碳和水的多重相互作用之间的协同性
对二甲基亚砜与二氧化碳和水分子的相互作用进行了彻底的研究,这些分子诱导了 18 种非常稳定的复合物。将 CO2 或 H2O 分子添加到 DMSO…1CO2 和 DMSO…1H2O 系统会导致所得复合物的稳定性增加,其中添加 H2O 的稳定性大于添加 CO2 的稳定性。DMSO⋯1,2CO2 的整体稳定能主要由 S=O⋯C Lewis 酸碱相互作用贡献,而 O − H⋯O 氢键在稳定 DMSO⋯1,2H2O 和DMSO⋯1CO2⋯1H2O。值得注意的是,DMSO⋯2H2O 的配合物被发现比 DMSO⋯1CO2⋯1H2O 和 DMSO⋯2CO2 更稳定。三元配合物中多重相互作用的协同性水平在从DMSO⋯2H2O到DMSO⋯1CO2⋯1H2O,最后到DMSO⋯2CO2的过程中趋于降低。通常发现,当发生协同效应时,O − H⋯O 氢键中涉及的O − H 键的红移增加,而C − H⋯O 氢键中的C − H 键的蓝移减少与相应的二元配合物相比,在三元配合物中。© 2018 Wiley Periodicals, Inc.
更新日期:2018-12-03
中文翻译:

深入了解二甲基亚砜与二氧化碳和水的多重相互作用之间的协同性
对二甲基亚砜与二氧化碳和水分子的相互作用进行了彻底的研究,这些分子诱导了 18 种非常稳定的复合物。将 CO2 或 H2O 分子添加到 DMSO…1CO2 和 DMSO…1H2O 系统会导致所得复合物的稳定性增加,其中添加 H2O 的稳定性大于添加 CO2 的稳定性。DMSO⋯1,2CO2 的整体稳定能主要由 S=O⋯C Lewis 酸碱相互作用贡献,而 O − H⋯O 氢键在稳定 DMSO⋯1,2H2O 和DMSO⋯1CO2⋯1H2O。值得注意的是,DMSO⋯2H2O 的配合物被发现比 DMSO⋯1CO2⋯1H2O 和 DMSO⋯2CO2 更稳定。三元配合物中多重相互作用的协同性水平在从DMSO⋯2H2O到DMSO⋯1CO2⋯1H2O,最后到DMSO⋯2CO2的过程中趋于降低。通常发现,当发生协同效应时,O − H⋯O 氢键中涉及的O − H 键的红移增加,而C − H⋯O 氢键中的C − H 键的蓝移减少与相应的二元配合物相比,在三元配合物中。© 2018 Wiley Periodicals, Inc.











































 京公网安备 11010802027423号
京公网安备 11010802027423号