当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Conversion of Activated 5‐Hydroxymethylfurfural into δ‐Lactone‐Fused Cyclopentenones
ChemSusChem ( IF 7.5 ) Pub Date : 2018-12-21 , DOI: 10.1002/cssc.201802537 Rafael F. A. Gomes 1 , Jaime A. S. Coelho 1 , Carlos A. M. Afonso 1
ChemSusChem ( IF 7.5 ) Pub Date : 2018-12-21 , DOI: 10.1002/cssc.201802537 Rafael F. A. Gomes 1 , Jaime A. S. Coelho 1 , Carlos A. M. Afonso 1
Affiliation
|
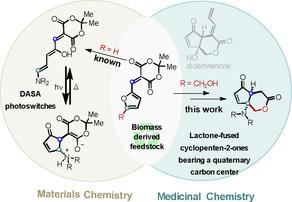
Valorization of biomass derived feedstock (e.g., 5‐hydroxymethylfurfural platform) is a very active field of chemical research. In this study, 5‐hydroxymethylfurfural is converted into cyclopenten‐2‐ones by virtue of furfural's activation and Meldrum's acid's tendency to undergo decomposition/esterification. Experimental and computational studies suggest a domino rearrangement–lactonization reaction involving BINOL‐catalyzed lactonization as the rate‐determining step. The novel lactone‐fused cyclopenten‐2‐ones, which bear a quaternary carbon and resemble a didemnenone natural product structure, are converted into several derivatives with potential interest for the fields of synthetic and medicinal chemistry.
中文翻译:

将活化的5-羟基甲基糠醛直接转化为δ-内酯融合的环戊烯酮
生物质衍生原料(例如5-羟甲基糠醛平台)的估价是化学研究的一个非常活跃的领域。在这项研究中,由于糠醛的活化和梅德鲁姆酸的分解/酯化趋势,5-羟甲基糠醛被转化为环戊烯-2-酮。实验和计算研究表明,以BINOL催化的内酯化为速率确定步骤的多米诺骨牌重排-内酯化反应。新型的内酯融合的环戊二烯-2-酮具有季碳原子,类似于二甲烯酮的天然产物结构,已被转化为几种衍生物,在合成化学和药物化学领域具有潜在的兴趣。
更新日期:2018-12-21
中文翻译:

将活化的5-羟基甲基糠醛直接转化为δ-内酯融合的环戊烯酮
生物质衍生原料(例如5-羟甲基糠醛平台)的估价是化学研究的一个非常活跃的领域。在这项研究中,由于糠醛的活化和梅德鲁姆酸的分解/酯化趋势,5-羟甲基糠醛被转化为环戊烯-2-酮。实验和计算研究表明,以BINOL催化的内酯化为速率确定步骤的多米诺骨牌重排-内酯化反应。新型的内酯融合的环戊二烯-2-酮具有季碳原子,类似于二甲烯酮的天然产物结构,已被转化为几种衍生物,在合成化学和药物化学领域具有潜在的兴趣。











































 京公网安备 11010802027423号
京公网安备 11010802027423号