当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Approaching Materials with Atomic Precision Using Supramolecular Cluster Assemblies
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-12-03 00:00:00 , DOI: 10.1021/acs.accounts.8b00369 Papri Chakraborty 1 , Abhijit Nag 1 , Amrita Chakraborty 1 , Thalappil Pradeep 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-12-03 00:00:00 , DOI: 10.1021/acs.accounts.8b00369 Papri Chakraborty 1 , Abhijit Nag 1 , Amrita Chakraborty 1 , Thalappil Pradeep 1
Affiliation
|
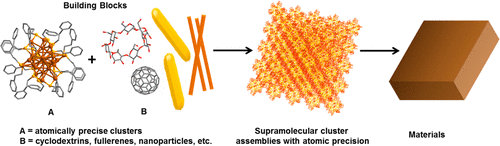
Supramolecular chemistry is a major area of chemistry that utilizes weaker non-covalent interactions between molecules, including hydrogen bonding, van der Waals, electrostatic, π···π, and C–H···π interactions. Such forces have been the basis of several molecular self-assemblies and host–guest complexes in organic, inorganic, and biological systems. Atomically precise nanoclusters (NCs) are materials of growing interest that display interesting structure–property correlations. The evolving science of such systems reaffirms their molecular behavior. This gives a possibility of exploring their supramolecular chemistry, leading to assemblies with similar or dissimilar cluster molecules. Such assemblies with compositional, structural, and conformational precision may ultimately result in cluster-assembled hybrid materials. In this Account, we present recent advancements on different possibilities of supramolecular interactions in atomically precise cluster systems that can occur at different length scales. We first present a brief discussion of the aspicule model of clusters, considering Au25(SR)18 as an example, that can explain various aspects of its atomic precision and distinguish the similar or dissimilar interacting sites in their structures. The supramolecular interaction of 4-tert-butylbenzyl mercaptan (BBSH)-protected [Au25(SBB)18]− NCs with cyclodextrins (CD) to form Au25SBB18∩CDn (n = 1–4) and that of [Ag29(BDT)12]3– with fullerenes to form [Ag29(BDT)12(C60)n]3– (n = 1–9) (BDT = 1,3-benzenedithiolate) are discussed subsequently. The formation of these adducts was studied by electrospray ionization mass spectrometry (ESI MS), optical absorption and NMR spectroscopy. In the subsequent sections, we discuss how variation in intercluster interactions can lead to polymorphic crystals, which are observable in single-crystal X-ray diffraction. Taking [Ag29(BDT)12(TPP)4]3– (TPP = triphenylphosphine) clusters as an example, we discuss how the different patterns of C–H···π and π···π interactions between the secondary ligands can alter the packing of the NCs into cubic and trigonal lattices. Finally, we discuss how the supramolecular interactions of atomically precise clusters can result in their hybrid assemblies with plasmonic nanostructures. The interaction of p-mercaptobenzoic acid (p-MBA)-protected Ag44(p-MBA)30 NCs with tellurium nanowires (Te NWs) can form crossed-bilayer precision assemblies with a woven-fabric-like structure with an angle of 81° between the layers. Similar crossed-bilayer assemblies show an angle of 77° when Au102(p-MBA)44 clusters are used to form the structure. Such assemblies were studied by transmission electron microscopy (TEM). Precision in these hybrid assemblies of Te NWs was highly controlled by the geometry of the ligands on the NC surface. Moreover, we also present how Ag44(p-MBA)30 clusters can encapsulate gold nanorods to form cage-like nanostructures. Such studies involved TEM, scanning transmission electron microscopy (STEM), and three-dimensional tomographic reconstructions of the nanostructures. The hydrogen bonding interactions of the −COOH groups of the p-MBA ligands were the major driving force in both of these cases. An important aspect that is central to the advancement of the area is the close interplay of molecular tools such as MS with structural tools such as TEM along with detailed computational modeling. We finally conclude this Account with a future perspective on the supramolecular chemistry of clusters. Advancements in this field will help in developing new materials with potential optical, electrical, and mechanical properties.
中文翻译:

使用超分子簇组件以原子精度接近材料
超分子化学是化学的主要领域,它利用分子之间较弱的非共价相互作用,包括氢键,范德华力,静电,π···π和C–H···π相互作用。这种力是有机,无机和生物系统中几种分子自组装体和客体-客体复合体的基础。原子精确的纳米团簇(NCs)是人们越来越感兴趣的材料,它们显示出有趣的结构-特性相关性。此类系统不断发展的科学再次证实了它们的分子行为。这提供了探索其超分子化学性质,从而导致具有相似或不相似簇分子的组装的可能性。具有组成,结构和构象精度的此类组装最终可能导致簇组装的混合材料。在这个帐户中,我们介绍了在原子精确的簇系统中可能发生在不同长度尺度上的超分子相互作用的不同可能性的最新进展。首先,我们考虑Au,简要介绍了集群的小规模模型。以图25(SR)18为例,它可以解释其原子精度的各个方面,并区分其结构中相似或不相似的相互作用位点。的超分子相互作用4-叔丁基苄基硫醇(BBSH)保护的[金25(SBB)18 ] -的NC与环糊精(CD),以形成金25 SBB 18 ∩CD Ñ(Ñ = 1-4)和其的[ Ag 29(BDT)12 ] 3–与富勒烯形成[Ag 29(BDT)12(C 60)n ] 3–(n = 1–9)(BDT = 1,3-苯二硫代磺酸盐)随后讨论。通过电喷雾电离质谱(ESI MS),光吸收和NMR光谱研究了这些加合物的形成。在接下来的部分中,我们将讨论簇间相互作用的变化如何导致多晶型晶体,这在单晶X射线衍射中可以观察到。服用[Ag 29(BDT)12(TPP)4 ] 3–以(TPP =三苯膦)簇为例,我们讨论了次级配体之间的C–H···π和π···π相互作用的不同模式如何改变NCs堆积成立方和三角晶格。最后,我们讨论原子精确团簇的超分子相互作用如何导致它们具有等离激元纳米结构的混合组装。对-巯基苯甲酸(p -MBA)保护的Ag 44(p -MBA)30 NC与碲纳米线(Te NWs)的相互作用可形成交叉双层精密组件,其结构类似于编织纤维结构,夹角为81层之间的°。当Au 102(p- MBA)44个簇用于形成该结构。通过透射电子显微镜(TEM)研究了此类组件。这些Te NW混合组件的精度受NC表面配体的几何形状高度控制。此外,我们还介绍了Ag 44(p -MBA)30团簇如何封装金纳米棒以形成笼状纳米结构。此类研究涉及TEM,扫描透射电子显微镜(STEM)和纳米结构的三维层析成像重建。p的-COOH基团的氢键相互作用-MBA配体是这两种情况的主要驱动力。对该领域的发展至关重要的一个重要方面是分子工具(例如MS)与结构工具(例如TEM)以及详细的计算模型之间的紧密相互作用。最后,我们以对簇的超分子化学的未来展望来结束本报告。该领域的进步将有助于开发具有潜在光学,电气和机械特性的新材料。
更新日期:2018-12-03
中文翻译:

使用超分子簇组件以原子精度接近材料
超分子化学是化学的主要领域,它利用分子之间较弱的非共价相互作用,包括氢键,范德华力,静电,π···π和C–H···π相互作用。这种力是有机,无机和生物系统中几种分子自组装体和客体-客体复合体的基础。原子精确的纳米团簇(NCs)是人们越来越感兴趣的材料,它们显示出有趣的结构-特性相关性。此类系统不断发展的科学再次证实了它们的分子行为。这提供了探索其超分子化学性质,从而导致具有相似或不相似簇分子的组装的可能性。具有组成,结构和构象精度的此类组装最终可能导致簇组装的混合材料。在这个帐户中,我们介绍了在原子精确的簇系统中可能发生在不同长度尺度上的超分子相互作用的不同可能性的最新进展。首先,我们考虑Au,简要介绍了集群的小规模模型。以图25(SR)18为例,它可以解释其原子精度的各个方面,并区分其结构中相似或不相似的相互作用位点。的超分子相互作用4-叔丁基苄基硫醇(BBSH)保护的[金25(SBB)18 ] -的NC与环糊精(CD),以形成金25 SBB 18 ∩CD Ñ(Ñ = 1-4)和其的[ Ag 29(BDT)12 ] 3–与富勒烯形成[Ag 29(BDT)12(C 60)n ] 3–(n = 1–9)(BDT = 1,3-苯二硫代磺酸盐)随后讨论。通过电喷雾电离质谱(ESI MS),光吸收和NMR光谱研究了这些加合物的形成。在接下来的部分中,我们将讨论簇间相互作用的变化如何导致多晶型晶体,这在单晶X射线衍射中可以观察到。服用[Ag 29(BDT)12(TPP)4 ] 3–以(TPP =三苯膦)簇为例,我们讨论了次级配体之间的C–H···π和π···π相互作用的不同模式如何改变NCs堆积成立方和三角晶格。最后,我们讨论原子精确团簇的超分子相互作用如何导致它们具有等离激元纳米结构的混合组装。对-巯基苯甲酸(p -MBA)保护的Ag 44(p -MBA)30 NC与碲纳米线(Te NWs)的相互作用可形成交叉双层精密组件,其结构类似于编织纤维结构,夹角为81层之间的°。当Au 102(p- MBA)44个簇用于形成该结构。通过透射电子显微镜(TEM)研究了此类组件。这些Te NW混合组件的精度受NC表面配体的几何形状高度控制。此外,我们还介绍了Ag 44(p -MBA)30团簇如何封装金纳米棒以形成笼状纳米结构。此类研究涉及TEM,扫描透射电子显微镜(STEM)和纳米结构的三维层析成像重建。p的-COOH基团的氢键相互作用-MBA配体是这两种情况的主要驱动力。对该领域的发展至关重要的一个重要方面是分子工具(例如MS)与结构工具(例如TEM)以及详细的计算模型之间的紧密相互作用。最后,我们以对簇的超分子化学的未来展望来结束本报告。该领域的进步将有助于开发具有潜在光学,电气和机械特性的新材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号