当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Prediction on the Origin of Selectivities in Base‐controlled Switchable NHC‐catalyzed Transformations
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-12-13 , DOI: 10.1002/asia.201801583 Yang Wang 1 , Ling‐Bo Qu 2 , Donghui Wei 2
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-12-13 , DOI: 10.1002/asia.201801583 Yang Wang 1 , Ling‐Bo Qu 2 , Donghui Wei 2
Affiliation
|
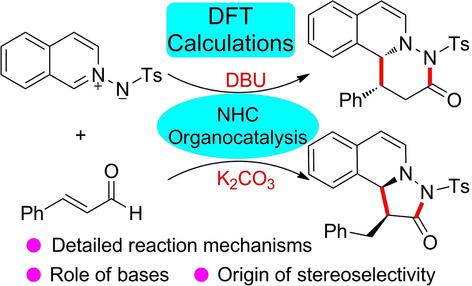
The base‐controlled mechanisms for N‐heterocyclic carbene (NHC)‐catalyzed divergent [3+3] and [3+2] annulation reactions were examined by using the DFT method. The reaction initiates with the complexation of NHC and enal to give the Breslow intermediate, which diverges afterward. Then, the azomethine imine can either react with the Breslow intermediate to give the six‐membered ring product or the β‐carbon protonation occurs for forming the enolate intermediate controlled by different bases. The formed enolate intermediate reacts with azomethine imine to afford the five‐membered ring product. The calculated results show that only the base K2CO3 can facilitate the structural transformation between homoenolate and enolate to switch the chemoselectivity; therefore, the [3+3] annulation happens preferentially in the presence of base DBU while the other situation occurs with K2CO3 as base. The NCI analysis results reveal that the stereoselectivity is predominately determined by the π⋅⋅⋅π, C−H⋅⋅⋅O, and C−H⋅⋅⋅N interactions. The obtained mechanistic insights should provide valuable clues for the rational design of these kinds of divergent reactions.
中文翻译:

在碱控制的可切换NHC催化的转化中的选择性起源的预测
使用DFT方法研究了N杂环卡宾(NHC)催化发散[3 + 3]和[3 + 2]环化反应的碱控制机理。该反应开始于NHC和烯醛的络合,生成Breslow中间体,此中间体随后发生分歧。然后,偶氮甲碱亚胺可与Breslow中间体反应生成六元环产物,或发生β-碳质子化反应以形成受不同碱控制的烯醇式中间体。形成的烯醇化物中间体与偶氮甲亚胺反应生成五元环产物。计算结果表明,只有碱K 2 CO 3可以促进均烯酸酯和烯醇酸酯之间的结构转变以切换化学选择性;因此,[3 + 3]环空优先在存在碱DBU的情况下发生,而其他情况则以K 2 CO 3为碱发生。NCI分析结果表明,立体选择性主要由π⋅⋅⋅π,CH-H⋅⋅⋅O和CH-H⋅⋅⋅N相互作用决定。所获得的力学见解应为合理设计此类发散反应提供有价值的线索。
更新日期:2018-12-13
中文翻译:

在碱控制的可切换NHC催化的转化中的选择性起源的预测
使用DFT方法研究了N杂环卡宾(NHC)催化发散[3 + 3]和[3 + 2]环化反应的碱控制机理。该反应开始于NHC和烯醛的络合,生成Breslow中间体,此中间体随后发生分歧。然后,偶氮甲碱亚胺可与Breslow中间体反应生成六元环产物,或发生β-碳质子化反应以形成受不同碱控制的烯醇式中间体。形成的烯醇化物中间体与偶氮甲亚胺反应生成五元环产物。计算结果表明,只有碱K 2 CO 3可以促进均烯酸酯和烯醇酸酯之间的结构转变以切换化学选择性;因此,[3 + 3]环空优先在存在碱DBU的情况下发生,而其他情况则以K 2 CO 3为碱发生。NCI分析结果表明,立体选择性主要由π⋅⋅⋅π,CH-H⋅⋅⋅O和CH-H⋅⋅⋅N相互作用决定。所获得的力学见解应为合理设计此类发散反应提供有价值的线索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号