当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization and Evaluation of Carbon-Supported Noble Metals for the Hydrodeoxygenation of Acetic Acid
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-11-30 00:00:00 , DOI: 10.1021/acs.oprd.8b00288 Jose Contreras-Mora 1 , Ritubarna Banerjee 1 , Brandon Bolton 1 , John Valentin 1 , John R. Monnier 1 , Christopher T. Williams 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-11-30 00:00:00 , DOI: 10.1021/acs.oprd.8b00288 Jose Contreras-Mora 1 , Ritubarna Banerjee 1 , Brandon Bolton 1 , John Valentin 1 , John R. Monnier 1 , Christopher T. Williams 1
Affiliation
|
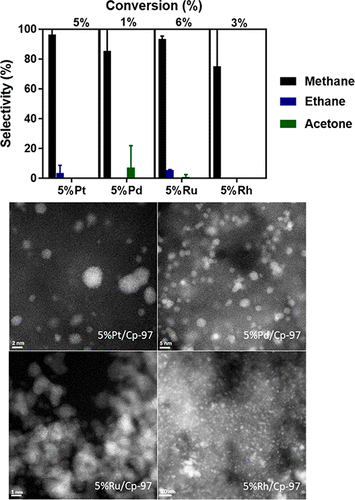
The gas-phase catalytic hydrodeoxygenation (HDO) of acetic acid (AA) over carbon-supported noble metals (5% M on Cp97, where M = Pt, Pd, Ru, or Rh) were studied. The temperature-dependent conversion and selectivity were studied at 1 atm from 200 to 400 °C. For Pt, Pd, and Rh, the main pathway from 200 to 300 °C was decarbonylation, and from 350 to 400 °C the main pathways were decarbonylation/decarboxylation and ketonization. For Ru, however, from 200 to 250 °C the main pathway was decarboxylation, and from 300 to 400 °C the main pathway was decarbonylation. The activity trend based on turnover frequency at 200 °C was found to be Ru > Rh ≈ Pt > Pd. The activities of all of the catalysts at 200 °C were found to decrease after reaction at 400 °C and a return to 200 °C. This is attributed to sintering and coking. The reaction orders in AA and H2 measured at 200 °C for all of the catalysts are generally well below ∼0.5, suggesting relatively strong adsorption of both reactants on all of the metal surfaces. The temperature dependence of the reaction rates was examined over the range 200–240 °C, and apparent activation energies of around 21 kcal/mol were found for all of the catalysts since decarbonylation/decarboxylation is the main pathway with similar product distribution.
中文翻译:

乙酸加氢脱氧的碳载贵金属的表征与评价
研究了碳负载贵金属(Cp97上的5%M,其中M = Pt,Pd,Ru或Rh)上乙酸(AA)的气相催化加氢脱氧(HDO)。在200至400°C的1个大气压下研究了随温度变化的转化率和选择性。对于Pt,Pd和Rh,从200到300°C的主要途径是脱羰,而从350到400°C的主要途径是脱羰/脱羧和酮化。但是,对于Ru,从200到250°C的主要途径是脱羧,而从300到400°C的主要途径是脱羰。在200°C下,基于翻转频率的活度趋势为Ru> Rh≈Pt> Pd。发现所有催化剂在200℃下的活性在400℃下反应后降低并返回至200℃。这归因于烧结和焦化。AA和H中的反应顺序2在200℃下对所有的催化剂的测量通常远低于〜0.5,表明对所有的金属表面的两种反应物的相对强的吸附。在200–240°C的温度范围内检查了反应速率的温度依赖性,发现所有催化剂的活化能约为21 kcal / mol,因为脱羰/脱羧是类似产物分布的主要途径。
更新日期:2018-11-30
中文翻译:

乙酸加氢脱氧的碳载贵金属的表征与评价
研究了碳负载贵金属(Cp97上的5%M,其中M = Pt,Pd,Ru或Rh)上乙酸(AA)的气相催化加氢脱氧(HDO)。在200至400°C的1个大气压下研究了随温度变化的转化率和选择性。对于Pt,Pd和Rh,从200到300°C的主要途径是脱羰,而从350到400°C的主要途径是脱羰/脱羧和酮化。但是,对于Ru,从200到250°C的主要途径是脱羧,而从300到400°C的主要途径是脱羰。在200°C下,基于翻转频率的活度趋势为Ru> Rh≈Pt> Pd。发现所有催化剂在200℃下的活性在400℃下反应后降低并返回至200℃。这归因于烧结和焦化。AA和H中的反应顺序2在200℃下对所有的催化剂的测量通常远低于〜0.5,表明对所有的金属表面的两种反应物的相对强的吸附。在200–240°C的温度范围内检查了反应速率的温度依赖性,发现所有催化剂的活化能约为21 kcal / mol,因为脱羰/脱羧是类似产物分布的主要途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号