当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sea Urchin Embryo Model As a Reliable in Vivo Phenotypic Screen to Characterize Selective Antimitotic Molecules. Comparative evaluation of Combretapyrazoles, -isoxazoles, -1,2,3-triazoles, and -pyrroles as Tubulin-Binding Agents
ACS Combinatorial Science Pub Date : 2018-11-19 00:00:00 , DOI: 10.1021/acscombsci.8b00113 Marina N. Semenova 1 , Dmitry V. Demchuk 2 , Dmitry V. Tsyganov 2 , Natalia B. Chernysheva 2 , Alexander V. Samet 2 , Eugenia A. Silyanova 2 , Victor P. Kislyi 2 , Anna S. Maksimenko 2 , Alexander E. Varakutin 2 , Leonid D. Konyushkin 2 , Mikhail M. Raihstat 2 , Alex S. Kiselyov 3 , Victor V. Semenov 2
ACS Combinatorial Science Pub Date : 2018-11-19 00:00:00 , DOI: 10.1021/acscombsci.8b00113 Marina N. Semenova 1 , Dmitry V. Demchuk 2 , Dmitry V. Tsyganov 2 , Natalia B. Chernysheva 2 , Alexander V. Samet 2 , Eugenia A. Silyanova 2 , Victor P. Kislyi 2 , Anna S. Maksimenko 2 , Alexander E. Varakutin 2 , Leonid D. Konyushkin 2 , Mikhail M. Raihstat 2 , Alex S. Kiselyov 3 , Victor V. Semenov 2
Affiliation
|
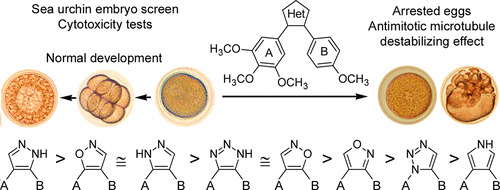
A series of both novel and reported combretastatin analogues, including diarylpyrazoles, -isoxazoles, -1,2,3-triazoles, and -pyrroles, were synthesized via improved protocols to evaluate their antimitotic antitubulin activity using in vivo sea urchin embryo assay and a panel of human cancer cells. A systematic comparative structure–activity relationship studies of these compounds were conducted. Pyrazoles 1i and 1p, isoxazole 3a, and triazole 7b were found to be the most potent antimitotics across all tested compounds causing cleavage alteration of the sea urchin embryo at 1, 0.25, 1, and 0.5 nM, respectively. These agents exhibited comparable cytotoxicity against human cancer cells. Structure–activity relationship studies revealed that compounds substituted with 3,4,5-trimethoxyphenyl ring A and 4-methoxyphenyl ring B displayed the highest activity. 3-Hydroxy group in the ring B was essential for the antiproliferative activity in the diarylisoxazole series, whereas it was not required for potency of diarylpyrazoles. Isoxazoles 3 with 3,4,5-trimethoxy-substituted ring A and 3-hydroxy-4-methoxy-substituted ring B were more active than the respective pyrazoles 1. Of the azoles substituted with the same set of other aryl pharmacophores, diarylpyrazoles 1, 4,5-diarylisoxazoles 3, and 4,5-diaryl-1,2,3-triazoles 7 displayed similar strongest antimitotic antitubulin effect followed by 3,4-diarylisoxazoles 5, 1,5-diaryl-1,2,3-triazoles 8, and pyrroles 10 that showed the lowest activity. Introduction of the amino group into the heterocyclic core decreased the antimitotic antitubulin effect of pyrazoles, triazoles, and to a lesser degree of 4,5-diarylisoxazoles, whereas potency of the respective 3,4-diarylisoxazoles was increased.
中文翻译:

海胆胚胎模型作为可靠的体内表型筛选来表征选择性抗有丝分裂分子。比较比较Combretapyrazoles,-isoxazoles,-1,2,3-triazoles和-pyrroles作为微管蛋白结合剂
通过改进的方案合成了一系列新颖的和已报道的康布雷他汀类似物,包括二芳基吡唑,-异恶唑,-1,2,3-三唑和-吡咯,以使用体内海胆胚胎测定法和专家小组评估其抗有丝分裂的抗微管蛋白活性。人类癌细胞 对这些化合物进行了系统的比较结构-活性关系研究。吡唑1i和1p,异恶唑3a和三唑7b被发现是所有测试化合物中最有效的抗有丝分裂剂,分别导致海胆胚胎的裂解改变分别为1、0.25、1和0.5 nM。这些试剂对人癌细胞显示出可比的细胞毒性。结构-活性关系研究表明,被3,4,5-三甲氧基苯基环A和4-甲氧基苯基环B取代的化合物表现出最高的活性。B环中的3-羟基对于二芳基异恶唑系列的抗增殖活性至关重要,而对二芳基吡唑的效力则不是必需的。具有3,4,5-三甲氧基取代的环A和3-羟基-4-甲氧基取代的环B的异恶唑3比各自的吡唑1具有更高的活性。与同一组的其他芳药效团取代的唑类,二芳基的1,4,5- diarylisoxazoles 3,和4,5-二芳基1,2,3-三唑7显示类似最强抗有丝分裂抗微管蛋白的效果,随后3,4- diarylisoxazoles 5,1,5-二芳基1,2,3-三唑8,和吡咯10的是显示出最低的活性。将氨基引入杂环核心降低了吡唑,三唑的抗有丝分裂抗微管蛋白作用,并降低了4,5-二芳基异恶唑的程度,而相应的3,4-二芳基异恶唑的效力增加了。
更新日期:2018-11-19
中文翻译:

海胆胚胎模型作为可靠的体内表型筛选来表征选择性抗有丝分裂分子。比较比较Combretapyrazoles,-isoxazoles,-1,2,3-triazoles和-pyrroles作为微管蛋白结合剂
通过改进的方案合成了一系列新颖的和已报道的康布雷他汀类似物,包括二芳基吡唑,-异恶唑,-1,2,3-三唑和-吡咯,以使用体内海胆胚胎测定法和专家小组评估其抗有丝分裂的抗微管蛋白活性。人类癌细胞 对这些化合物进行了系统的比较结构-活性关系研究。吡唑1i和1p,异恶唑3a和三唑7b被发现是所有测试化合物中最有效的抗有丝分裂剂,分别导致海胆胚胎的裂解改变分别为1、0.25、1和0.5 nM。这些试剂对人癌细胞显示出可比的细胞毒性。结构-活性关系研究表明,被3,4,5-三甲氧基苯基环A和4-甲氧基苯基环B取代的化合物表现出最高的活性。B环中的3-羟基对于二芳基异恶唑系列的抗增殖活性至关重要,而对二芳基吡唑的效力则不是必需的。具有3,4,5-三甲氧基取代的环A和3-羟基-4-甲氧基取代的环B的异恶唑3比各自的吡唑1具有更高的活性。与同一组的其他芳药效团取代的唑类,二芳基的1,4,5- diarylisoxazoles 3,和4,5-二芳基1,2,3-三唑7显示类似最强抗有丝分裂抗微管蛋白的效果,随后3,4- diarylisoxazoles 5,1,5-二芳基1,2,3-三唑8,和吡咯10的是显示出最低的活性。将氨基引入杂环核心降低了吡唑,三唑的抗有丝分裂抗微管蛋白作用,并降低了4,5-二芳基异恶唑的程度,而相应的3,4-二芳基异恶唑的效力增加了。











































 京公网安备 11010802027423号
京公网安备 11010802027423号