当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual Role of N‐Bromosuccinimide as Oxidant and Succinimide Surrogate in Domino One‐Pot Oxidative Amination of Benzyl Alcohols for the Synthesis of α–Imido Ketones
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-11-28 , DOI: 10.1002/slct.201803465 Madithedu Muneeswara 1 , Alagesan Muthukumar 1 , Govindasamy Sekar 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-11-28 , DOI: 10.1002/slct.201803465 Madithedu Muneeswara 1 , Alagesan Muthukumar 1 , Govindasamy Sekar 1
Affiliation
|
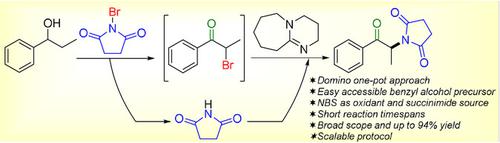
A transition‐metal‐free oxidative amination of secondary benzyl alcohols has been developed using N‐bromosuccinimide as the oxidant and source of succinimide. The control experiments revealed that the transformation underwent via oxidation of alcohol to ketone, α‐bromination of ketone and diazabicyclo[5.4.1]undec‐7‐ene (DBU) base‐mediated nucleophilic substitution of the by‐product succinimide. Various substituted benzyl alcohols and N‐bromoimides were examined to form α‐imido ketones in short reaction timespans. The protocol was successfully extended to a gram scale reaction.
中文翻译:

N-溴代琥珀酰亚胺作为氧化剂和琥珀酰亚胺替代物在苄醇的多米诺一锅氧化胺合成α-亚氨基酮中的双重作用
使用N-溴代琥珀酰亚胺作为琥珀酰亚胺的氧化剂和来源,开发了一种无过渡金属的仲苄醇氧化胺化方法。对照实验表明,转化是通过醇氧化为酮,酮的α-溴化和副产物琥珀酰亚胺的二氮杂双环[5.4.1]十一碳-7烯(DBU)碱基介导的亲核取代而进行的。在短的反应时间内检查了各种取代的苄醇和N-溴酰亚胺形成的α-亚氨基酮。该协议已成功扩展到克级反应。
更新日期:2018-11-28
中文翻译:

N-溴代琥珀酰亚胺作为氧化剂和琥珀酰亚胺替代物在苄醇的多米诺一锅氧化胺合成α-亚氨基酮中的双重作用
使用N-溴代琥珀酰亚胺作为琥珀酰亚胺的氧化剂和来源,开发了一种无过渡金属的仲苄醇氧化胺化方法。对照实验表明,转化是通过醇氧化为酮,酮的α-溴化和副产物琥珀酰亚胺的二氮杂双环[5.4.1]十一碳-7烯(DBU)碱基介导的亲核取代而进行的。在短的反应时间内检查了各种取代的苄醇和N-溴酰亚胺形成的α-亚氨基酮。该协议已成功扩展到克级反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号