当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nickel Boride Mediated Chemoselective Reduction of Aryldiazonium Tetrafluoroborates to Corresponding Aryl Hydrazines and Aryl Amines at Ambient Temperature.
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-11-28 , DOI: 10.1002/slct.201802436 Gaurav Bartwal 1 , Mohit Saroha 1 , Jitender M. Khurana 1
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-11-28 , DOI: 10.1002/slct.201802436 Gaurav Bartwal 1 , Mohit Saroha 1 , Jitender M. Khurana 1
Affiliation
|
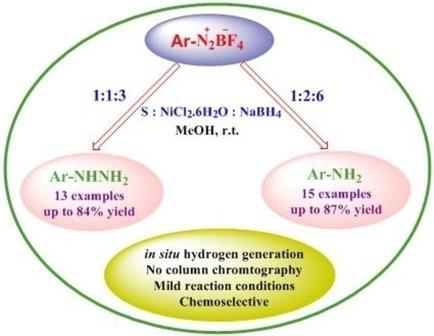
An efficient method has been reported for the chemoselective reduction of aryl diazonium tetrafluoroborates to corresponding hydrazines or anilines with nickel boride generated in situ from nickel chloride and sodium borohydride at room temperature using 1:1:3 or 1:2:6 molar ratio of substrate : NiCl2 : NaBH4. The aryl amines are obtained by in situ reduction of aryl hydrazines formed initially. Halo, carboxy, carboethoxy and methoxy groups remain unaffected under these conditions. Mild reaction conditions, easy work‐up and chemoselectivity are major advantages of this new procedure.
中文翻译:

在环境温度下,硼化镍介导的四氟硼酸芳重氮鎓化学选择性还原成相应的芳基肼和芳基胺。
已经报道了一种有效方法,该方法是在室温下使用摩尔比为1:1:3或1:2:6的氯化镍和硼氢化钠原位生成的硼化镍将四氟硼酸芳基重氮鎓化学选择性还原为相应的肼或苯胺。 :NiCl 2:NaBH 4。通过原位还原最初形成的芳基肼获得芳基胺。在这些条件下,卤代,羧基,碳乙氧基和甲氧基保持不受影响。温和的反应条件,容易的后处理和化学选择性是该新方法的主要优点。
更新日期:2018-11-28
中文翻译:

在环境温度下,硼化镍介导的四氟硼酸芳重氮鎓化学选择性还原成相应的芳基肼和芳基胺。
已经报道了一种有效方法,该方法是在室温下使用摩尔比为1:1:3或1:2:6的氯化镍和硼氢化钠原位生成的硼化镍将四氟硼酸芳基重氮鎓化学选择性还原为相应的肼或苯胺。 :NiCl 2:NaBH 4。通过原位还原最初形成的芳基肼获得芳基胺。在这些条件下,卤代,羧基,碳乙氧基和甲氧基保持不受影响。温和的反应条件,容易的后处理和化学选择性是该新方法的主要优点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号