Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metabolic reprogramming by the S-nitroso-CoA reductase system protects against kidney injury
Nature ( IF 50.5 ) Pub Date : 2018-11-28 , DOI: 10.1038/s41586-018-0749-z Hua-Lin Zhou 1 , Rongli Zhang 1 , Puneet Anand 1 , Colin T Stomberski 1 , Zhaoxia Qian 1 , Alfred Hausladen 1 , Liwen Wang 2 , Eugene P Rhee 3, 4 , Samir M Parikh 5, 6 , S Ananth Karumanchi 5, 6, 7 , Jonathan S Stamler 1, 8
Nature ( IF 50.5 ) Pub Date : 2018-11-28 , DOI: 10.1038/s41586-018-0749-z Hua-Lin Zhou 1 , Rongli Zhang 1 , Puneet Anand 1 , Colin T Stomberski 1 , Zhaoxia Qian 1 , Alfred Hausladen 1 , Liwen Wang 2 , Eugene P Rhee 3, 4 , Samir M Parikh 5, 6 , S Ananth Karumanchi 5, 6, 7 , Jonathan S Stamler 1, 8
Affiliation
|
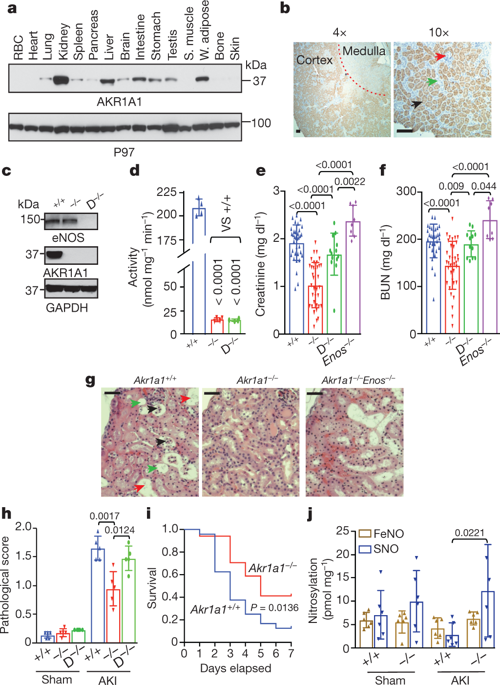
Endothelial nitric oxide synthase (eNOS) is protective against kidney injury, but the molecular mechanisms of this protection are poorly understood1,2. Nitric oxide-based cellular signalling is generally mediated by protein S-nitrosylation, the oxidative modification of Cys residues to form S-nitrosothiols (SNOs). S-nitrosylation regulates proteins in all functional classes, and is controlled by enzymatic machinery that includes S-nitrosylases and denitrosylases, which add and remove SNO from proteins, respectively3,4. In Saccharomyces cerevisiae, the classic metabolic intermediate co-enzyme A (CoA) serves as an endogenous source of SNOs through its conjugation with nitric oxide to form S-nitroso-CoA (SNO-CoA), and S-nitrosylation of proteins by SNO-CoA is governed by its cognate denitrosylase, SNO-CoA reductase (SCoR)5. Mammals possess a functional homologue of yeast SCoR, an aldo-keto reductase family member (AKR1A1)5 with an unknown physiological role. Here we report that the SNO-CoA–AKR1A1 system is highly expressed in renal proximal tubules, where it transduces the activity of eNOS in reprogramming intermediary metabolism, thereby protecting kidneys against acute kidney injury. Specifically, deletion of Akr1a1 in mice to reduce SCoR activity increased protein S-nitrosylation, protected against acute kidney injury and improved survival, whereas this protection was lost when Enos (also known as Nos3) was also deleted. Metabolic profiling coupled with unbiased mass spectrometry-based SNO-protein identification revealed that protection by the SNO-CoA–SCoR system is mediated by inhibitory S-nitrosylation of pyruvate kinase M2 (PKM2) through a novel locus of regulation, thereby balancing fuel utilization (through glycolysis) with redox protection (through the pentose phosphate shunt). Targeted deletion of PKM2 from mouse proximal tubules recapitulated precisely the protective and mechanistic effects of S-nitrosylation in Akr1a1−/− mice, whereas Cys-mutant PKM2, which is refractory to S-nitrosylation, negated SNO-CoA bioactivity. Our results identify a physiological function of the SNO-CoA–SCoR system in mammals, describe new regulation of renal metabolism and of PKM2 in differentiated tissues, and offer a novel perspective on kidney injury with therapeutic implications.AKR1A1-regulated protein S-nitrosylation protects against kidney injury through PKM2-mediated metabolic reprogramming.
中文翻译:

S-亚硝基辅酶A还原酶系统的代谢重编程可防止肾损伤
内皮一氧化氮合酶 (eNOS) 对肾损伤具有保护作用,但对这种保护作用的分子机制知之甚少1,2。基于一氧化氮的细胞信号传导通常由蛋白质 S-亚硝基化介导,即 Cys 残基的氧化修饰形成 S-亚硝基硫醇 (SNO)。S-亚硝基化调节所有功能类别的蛋白质,并由包括 S-亚硝基酶和脱硝基酶在内的酶机制控制,它们分别从蛋白质中添加和去除 SNO3,4。在酿酒酵母中,经典的代谢中间体辅酶 A (CoA) 通过与一氧化氮结合形成 S-亚硝基-CoA (SNO-CoA) 和 SNO- 对蛋白质进行 S-亚硝基化,作为 SNO 的内源性来源。 CoA 受其同源脱亚硝基酶 SNO-CoA 还原酶 (SCoR)5 控制。哺乳动物具有酵母 SCoR 的功能同源物,酵母 SCoR 是一种具有未知生理作用的醛酮还原酶家族成员 (AKR1A1)5。在这里,我们报告 SNO-CoA-AKR1A1 系统在肾近端小管中高度表达,在那里它转导 eNOS 在重编程中间代谢中的活性,从而保护肾脏免受急性肾损伤。具体而言,在小鼠中删除 Akr1a1 以降低 SCoR 活性会增加蛋白质 S-亚硝基化,从而防止急性肾损伤并提高存活率,而当 Enos(也称为 Nos3)也被删除时,这种保护作用就会消失。代谢分析与基于无偏质谱的 SNO 蛋白鉴定相结合,表明 SNO-CoA-SCoR 系统的保护作用是由丙酮酸激酶 M2 (PKM2) 的抑制性 S-亚硝基化通过新的调控位点介导的,从而平衡燃料利用。通过糖酵解)具有氧化还原保护(通过磷酸戊糖分流)。从小鼠近端小管中靶向缺失 PKM2 精确地概括了 Akr1a1-/- 小鼠中 S-亚硝基化的保护和机械作用,而对 S-亚硝基化无效的 Cys 突变体 PKM2 否定了 SNO-CoA 生物活性。我们的结果确定了哺乳动物中 SNO-CoA-SCoR 系统的生理功能,描述了肾脏代谢和分化组织中 PKM2 的新调节,
更新日期:2018-11-28
中文翻译:

S-亚硝基辅酶A还原酶系统的代谢重编程可防止肾损伤
内皮一氧化氮合酶 (eNOS) 对肾损伤具有保护作用,但对这种保护作用的分子机制知之甚少1,2。基于一氧化氮的细胞信号传导通常由蛋白质 S-亚硝基化介导,即 Cys 残基的氧化修饰形成 S-亚硝基硫醇 (SNO)。S-亚硝基化调节所有功能类别的蛋白质,并由包括 S-亚硝基酶和脱硝基酶在内的酶机制控制,它们分别从蛋白质中添加和去除 SNO3,4。在酿酒酵母中,经典的代谢中间体辅酶 A (CoA) 通过与一氧化氮结合形成 S-亚硝基-CoA (SNO-CoA) 和 SNO- 对蛋白质进行 S-亚硝基化,作为 SNO 的内源性来源。 CoA 受其同源脱亚硝基酶 SNO-CoA 还原酶 (SCoR)5 控制。哺乳动物具有酵母 SCoR 的功能同源物,酵母 SCoR 是一种具有未知生理作用的醛酮还原酶家族成员 (AKR1A1)5。在这里,我们报告 SNO-CoA-AKR1A1 系统在肾近端小管中高度表达,在那里它转导 eNOS 在重编程中间代谢中的活性,从而保护肾脏免受急性肾损伤。具体而言,在小鼠中删除 Akr1a1 以降低 SCoR 活性会增加蛋白质 S-亚硝基化,从而防止急性肾损伤并提高存活率,而当 Enos(也称为 Nos3)也被删除时,这种保护作用就会消失。代谢分析与基于无偏质谱的 SNO 蛋白鉴定相结合,表明 SNO-CoA-SCoR 系统的保护作用是由丙酮酸激酶 M2 (PKM2) 的抑制性 S-亚硝基化通过新的调控位点介导的,从而平衡燃料利用。通过糖酵解)具有氧化还原保护(通过磷酸戊糖分流)。从小鼠近端小管中靶向缺失 PKM2 精确地概括了 Akr1a1-/- 小鼠中 S-亚硝基化的保护和机械作用,而对 S-亚硝基化无效的 Cys 突变体 PKM2 否定了 SNO-CoA 生物活性。我们的结果确定了哺乳动物中 SNO-CoA-SCoR 系统的生理功能,描述了肾脏代谢和分化组织中 PKM2 的新调节,











































 京公网安备 11010802027423号
京公网安备 11010802027423号