Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells
Nature ( IF 50.5 ) Pub Date : 2018-11-28 , DOI: 10.1038/s41586-018-0756-0 Xiangbo Meng , Xiwei Liu , Xingdong Guo , Shutan Jiang , Tingting Chen , Zhiqiang Hu , Haifeng Liu , Yibing Bai , Manman Xue , Ronggui Hu , Shao-cong Sun , Xiaolong Liu , Penghui Zhou , Xiaowu Huang , Lai Wei , Wei Yang , Chenqi Xu
Nature ( IF 50.5 ) Pub Date : 2018-11-28 , DOI: 10.1038/s41586-018-0756-0 Xiangbo Meng , Xiwei Liu , Xingdong Guo , Shutan Jiang , Tingting Chen , Zhiqiang Hu , Haifeng Liu , Yibing Bai , Manman Xue , Ronggui Hu , Shao-cong Sun , Xiaolong Liu , Penghui Zhou , Xiaowu Huang , Lai Wei , Wei Yang , Chenqi Xu
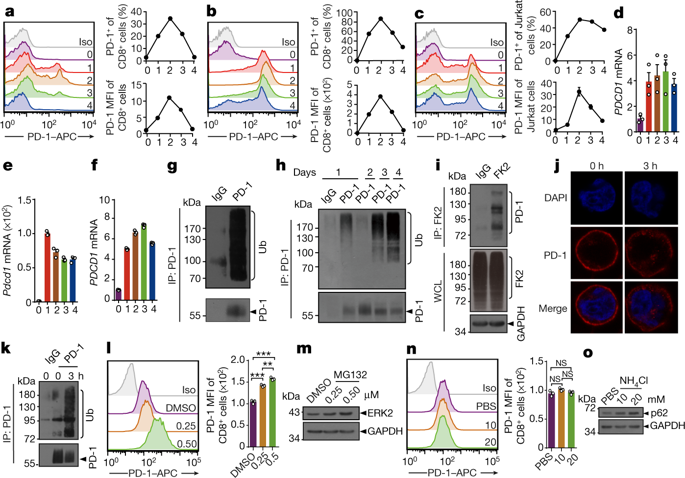
|
Dysfunctional T cells in the tumour microenvironment have abnormally high expression of PD-1 and antibody inhibitors against PD-1 or its ligand (PD-L1) have become commonly used drugs to treat various types of cancer1–4. The clinical success of these inhibitors highlights the need to study the mechanisms by which PD-1 is regulated. Here we report a mechanism of PD-1 degradation and the importance of this mechanism in anti-tumour immunity in preclinical models. We show that surface PD-1 undergoes internalization, subsequent ubiquitination and proteasome degradation in activated T cells. FBXO38 is an E3 ligase of PD-1 that mediates Lys48-linked poly-ubiquitination and subsequent proteasome degradation. Conditional knockout of Fbxo38 in T cells did not affect T cell receptor and CD28 signalling, but led to faster tumour progression in mice owing to higher levels of PD-1 in tumour-infiltrating T cells. Anti-PD-1 therapy normalized the effect of FBXO38 deficiency on tumour growth in mice, which suggests that PD-1 is the primary target of FBXO38 in T cells. In human tumour tissues and a mouse cancer model, transcriptional levels of FBXO38 and Fbxo38, respectively, were downregulated in tumour-infiltrating T cells. However, IL-2 therapy rescued Fbxo38 transcription and therefore downregulated PD-1 levels in PD-1+ T cells in mice. These data indicate that FBXO38 regulates PD-1 expression and highlight an alternative method to block the PD-1 pathway.PD-1 undergoes internalization, FBXO38-mediated ubiquitination and proteasome degradation in activated T cells, and inhibition of this pathway dampens anti-tumour immunity of T cells.
中文翻译:

FBXO38介导PD-1泛素化并调节T细胞的抗肿瘤免疫
肿瘤微环境中功能失调的 T 细胞异常高表达 PD-1,针对 PD-1 或其配体 (PD-L1) 的抗体抑制剂已成为治疗各种类型癌症的常用药物 1-4。这些抑制剂的临床成功凸显了研究 PD-1 调节机制的必要性。在这里,我们报告了 PD-1 降解的机制以及该机制在临床前模型中抗肿瘤免疫中的重要性。我们显示表面 PD-1 在活化的 T 细胞中经历内化、随后的泛素化和蛋白酶体降解。FBXO38 是 PD-1 的 E3 连接酶,可介导 Lys48 连接的多泛素化和随后的蛋白酶体降解。T 细胞中 Fbxo38 的条件性敲除不影响 T 细胞受体和 CD28 信号传导,但由于肿瘤浸润 T 细胞中更高水平的 PD-1,导致小鼠肿瘤进展更快。抗 PD-1 治疗使 FBXO38 缺乏对小鼠肿瘤生长的影响正常化,这表明 PD-1 是 T 细胞中 FBXO38 的主要靶点。在人类肿瘤组织和小鼠癌症模型中,FBXO38 和 Fbxo38 的转录水平分别在肿瘤浸润性 T 细胞中下调。然而,IL-2 疗法挽救了 Fbxo38 转录,因此下调了小鼠 PD-1+ T 细胞中的 PD-1 水平。这些数据表明 FBXO38 调节 PD-1 表达并突出了阻断 PD-1 通路的替代方法。 PD-1 在活化的 T 细胞中经历内化、FBXO38 介导的泛素化和蛋白酶体降解,抑制该通路会抑制抗肿瘤T细胞免疫。
更新日期:2018-11-28
中文翻译:

FBXO38介导PD-1泛素化并调节T细胞的抗肿瘤免疫
肿瘤微环境中功能失调的 T 细胞异常高表达 PD-1,针对 PD-1 或其配体 (PD-L1) 的抗体抑制剂已成为治疗各种类型癌症的常用药物 1-4。这些抑制剂的临床成功凸显了研究 PD-1 调节机制的必要性。在这里,我们报告了 PD-1 降解的机制以及该机制在临床前模型中抗肿瘤免疫中的重要性。我们显示表面 PD-1 在活化的 T 细胞中经历内化、随后的泛素化和蛋白酶体降解。FBXO38 是 PD-1 的 E3 连接酶,可介导 Lys48 连接的多泛素化和随后的蛋白酶体降解。T 细胞中 Fbxo38 的条件性敲除不影响 T 细胞受体和 CD28 信号传导,但由于肿瘤浸润 T 细胞中更高水平的 PD-1,导致小鼠肿瘤进展更快。抗 PD-1 治疗使 FBXO38 缺乏对小鼠肿瘤生长的影响正常化,这表明 PD-1 是 T 细胞中 FBXO38 的主要靶点。在人类肿瘤组织和小鼠癌症模型中,FBXO38 和 Fbxo38 的转录水平分别在肿瘤浸润性 T 细胞中下调。然而,IL-2 疗法挽救了 Fbxo38 转录,因此下调了小鼠 PD-1+ T 细胞中的 PD-1 水平。这些数据表明 FBXO38 调节 PD-1 表达并突出了阻断 PD-1 通路的替代方法。 PD-1 在活化的 T 细胞中经历内化、FBXO38 介导的泛素化和蛋白酶体降解,抑制该通路会抑制抗肿瘤T细胞免疫。










































 京公网安备 11010802027423号
京公网安备 11010802027423号