当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improvements to Enable the Large Scale Synthesis of 1-{[(2S,3S,4S)-3-Ethyl-4-fluoro-5-oxopyrrolidin-2-yl]methoxy}-7-methoxyisoquinoline-6-carboxamide (PF-06650833)
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-11-27 00:00:00 , DOI: 10.1021/acs.oprd.8b00386 Stephen W. Wright 1 , Bryan Li 2 , Zhihui Peng 2 , Lulin Wei 2 , Emma McInturff 2 , David Place 2 , David B. Damon 2 , Robert A. Singer 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-11-27 00:00:00 , DOI: 10.1021/acs.oprd.8b00386 Stephen W. Wright 1 , Bryan Li 2 , Zhihui Peng 2 , Lulin Wei 2 , Emma McInturff 2 , David Place 2 , David B. Damon 2 , Robert A. Singer 2
Affiliation
|
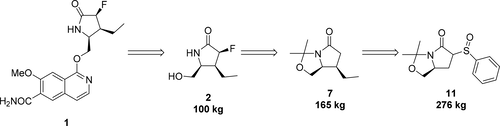
An improved process for the large scale synthesis of 1-{[(2S,3S,4S)-3-ethyl-4-fluoro-5-oxopyrrolidin-2-yl]methoxy}-7-methoxyisoquinoline-6-carboxamide (1), a candidate currently in clinical development, was developed. Key objectives were to eliminate chromatographic purifications, to maximize the reproducibility of each step, and to improve the yield and efficiency of each step relative to the previous discovery syntheses of 1. This work was focused on improvements to the synthesis of the stereochemically complex lactam 2. Steps of particular concern were the preparation of the unsaturated lactam 6, the cuprate conjugate addition reaction to produce 7, and the conversion of 7 to 8 with a high degree of diastereoselection. The solutions to these challenges have permitted the synthesis of 2 in excess of 100 kg, which in turn has permitted 1 to be prepared in sufficient amounts to support further development.
中文翻译:

进行改进以实现大规模合成1-{[(2S,3S,4S)-3-乙基-4-氟-5-氧吡咯烷-2--2-基]甲氧基} -7-甲氧基异喹啉-6-羧酰胺(PF-06650833 )
大规模合成1-{[(2S,3S,4S)-3-乙基-4-氟-5-氧吡咯烷基-2-基]甲氧基} -7-甲氧基异喹啉-6-羧酰胺的改进方法(1)已开发出目前正在临床开发中的候选药物。关键目标是消除色谱纯化,以最大化每个步骤的再现性,并提高每个步骤相对的先前发现的合成的产率和效率1。这项工作的重点是改进立体化学复合内酰胺2的合成。特别需要注意的步骤是不饱和内酰胺6的制备,与铜酸盐的共轭加成反应以生成7以及将7转化为8具有很高的非选择性。这些挑战的解决方案允许的合成2在超过100公斤,这反过来又具有允许1以足够的量被制备,以支持进一步的发展。
更新日期:2018-11-27
中文翻译:

进行改进以实现大规模合成1-{[(2S,3S,4S)-3-乙基-4-氟-5-氧吡咯烷-2--2-基]甲氧基} -7-甲氧基异喹啉-6-羧酰胺(PF-06650833 )
大规模合成1-{[(2S,3S,4S)-3-乙基-4-氟-5-氧吡咯烷基-2-基]甲氧基} -7-甲氧基异喹啉-6-羧酰胺的改进方法(1)已开发出目前正在临床开发中的候选药物。关键目标是消除色谱纯化,以最大化每个步骤的再现性,并提高每个步骤相对的先前发现的合成的产率和效率1。这项工作的重点是改进立体化学复合内酰胺2的合成。特别需要注意的步骤是不饱和内酰胺6的制备,与铜酸盐的共轭加成反应以生成7以及将7转化为8具有很高的非选择性。这些挑战的解决方案允许的合成2在超过100公斤,这反过来又具有允许1以足够的量被制备,以支持进一步的发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号