当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conversion of 2,4,6-Trimethylaniline to 3-(Mesitylthio)-1H-1,2,4-triazole Using a Continuous-Flow Reactor
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-11-26 00:00:00 , DOI: 10.1021/acs.oprd.8b00362 Zhiqun Yu , Jianyang Chen , Jiming Liu , Zhengkang Wu , Weike Su
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-11-26 00:00:00 , DOI: 10.1021/acs.oprd.8b00362 Zhiqun Yu , Jianyang Chen , Jiming Liu , Zhengkang Wu , Weike Su
|
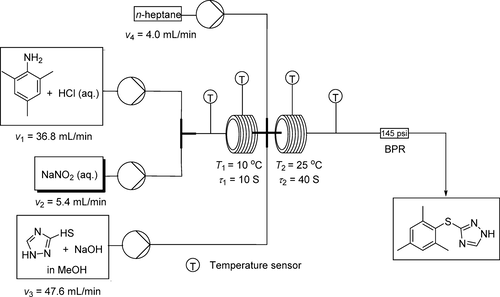
An expeditious and highly efficient continuous-flow process was developed for the synthesis of 3-(mesitylthio)-1H-1,2,4-triazole. The starting material 2,4,6-trimethylaniline was diazotized to give diazonium chloride, followed by azo-coupling with 1H-1,2,4-triazole-3-thiol and dediazoniation to provide 3-(mesitylthio)-1H-1,2,4-triazole in 85% yield with a productivity of 344 g/h. The side reactions such as hydrolysis, dimerization, and intramolecular coupling were significantly inhibited by the continuous-flow technology.
中文翻译:

使用连续流反应器将2,4,6-三甲基苯胺转化为3-(间甲硫基)-1 H -1,2,4-三唑
开发了一种快速且高效的连续流方法,用于合成3-(间甲硫基)-1 H -1,2,4-三唑。起始原料2,4,6-三甲基苯胺重氮化,得到重氮氯化物,接着偶氮耦合用1个ħ -1,2,4-三唑-3-硫醇和dediazoniation以提供3-(mesitylthio)-1 ħ - 1,2,4-三唑的产率为85%,生产率为344 g / h。连续流动技术显着抑制了诸如水解,二聚化和分子内偶联等副反应。
更新日期:2018-11-26
中文翻译:

使用连续流反应器将2,4,6-三甲基苯胺转化为3-(间甲硫基)-1 H -1,2,4-三唑
开发了一种快速且高效的连续流方法,用于合成3-(间甲硫基)-1 H -1,2,4-三唑。起始原料2,4,6-三甲基苯胺重氮化,得到重氮氯化物,接着偶氮耦合用1个ħ -1,2,4-三唑-3-硫醇和dediazoniation以提供3-(mesitylthio)-1 ħ - 1,2,4-三唑的产率为85%,生产率为344 g / h。连续流动技术显着抑制了诸如水解,二聚化和分子内偶联等副反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号