当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterisation of the biosynthetic pathway to agnestins A and B reveals the reductive route to chrysophanol in fungi.
Chemical Science ( IF 7.6 ) Pub Date : 2018-11-26 , DOI: 10.1039/c8sc03778g Agnieszka J Szwalbe 1 , Katherine Williams 1 , Zhongshu Song 1 , Kate de Mattos-Shipley 1 , Jason L Vincent 2 , Andrew M Bailey 3 , Christine L Willis 1 , Russell J Cox 1, 4, 5 , Thomas J Simpson 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-11-26 , DOI: 10.1039/c8sc03778g Agnieszka J Szwalbe 1 , Katherine Williams 1 , Zhongshu Song 1 , Kate de Mattos-Shipley 1 , Jason L Vincent 2 , Andrew M Bailey 3 , Christine L Willis 1 , Russell J Cox 1, 4, 5 , Thomas J Simpson 1
Affiliation
|
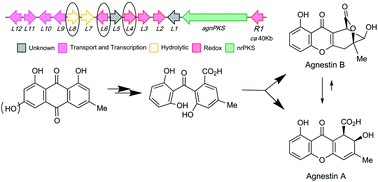
Two new dihydroxy-xanthone metabolites, agnestins A and B, were isolated from Paecilomyces variotii along with a number of related benzophenones and xanthones including monodictyphenone. The structures were elucidated by NMR analyses and X-ray crystallography. The agnestin (agn) biosynthetic gene cluster was identified and targeted gene disruptions of the PKS, Baeyer-Villiger monooxygenase, and other oxido-reductase genes revealed new details of fungal xanthone biosynthesis. In particular, identification of a reductase responsible for in vivo anthraquinone to anthrol conversion confirms a previously postulated essential step in aromatic deoxygenation of anthraquinones, e.g. emodin to chrysophanol.
中文翻译:

Agnestin A 和 B 生物合成途径的表征揭示了真菌中大黄酚的还原途径。
从拟青霉中分离出两种新的二羟基呫吨酮代谢物,agnestins A 和 B,以及许多相关的二苯甲酮和呫吨酮,包括单二苯酮。通过NMR分析和X射线晶体学阐明了结构。鉴定了 agnestin (agn) 生物合成基因簇,并对 PKS、Baeyer-Villiger 单加氧酶和其他氧化还原酶基因进行靶向基因破坏,揭示了真菌呫吨酮生物合成的新细节。特别地,负责体内蒽醌向蒽酚转化的还原酶的鉴定证实了先前假设的蒽醌芳香族脱氧作用(例如大黄素向大黄酚)中的必要步骤。
更新日期:2018-11-26
中文翻译:

Agnestin A 和 B 生物合成途径的表征揭示了真菌中大黄酚的还原途径。
从拟青霉中分离出两种新的二羟基呫吨酮代谢物,agnestins A 和 B,以及许多相关的二苯甲酮和呫吨酮,包括单二苯酮。通过NMR分析和X射线晶体学阐明了结构。鉴定了 agnestin (agn) 生物合成基因簇,并对 PKS、Baeyer-Villiger 单加氧酶和其他氧化还原酶基因进行靶向基因破坏,揭示了真菌呫吨酮生物合成的新细节。特别地,负责体内蒽醌向蒽酚转化的还原酶的鉴定证实了先前假设的蒽醌芳香族脱氧作用(例如大黄素向大黄酚)中的必要步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号