当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactions of Quinones—Mechanisms, Structures, and Prospects for Food Research
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2018-11-25 00:00:00 , DOI: 10.1021/acs.jafc.8b05215 Andreas Schieber 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2018-11-25 00:00:00 , DOI: 10.1021/acs.jafc.8b05215 Andreas Schieber 1
Affiliation
|
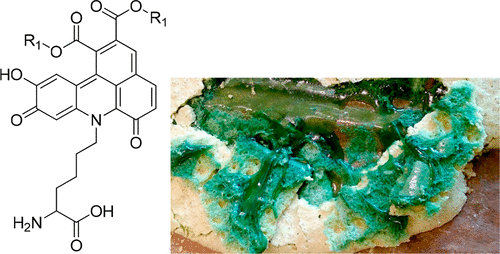
Oxidation of plant phenolics leads to quinones, which are unstable intermediates that may react with nucleophiles. Quinones play an important role in the enzymatic browning of fruits and vegetables and may form covalent adducts with amino acids, peptides, and proteins. These reactions may alter both the physicochemical and immunological properties of food proteins. Quinones trap odoriferous compounds and contribute to the formation of aroma compounds through Strecker degradation of amino acids. Oxidative dimerization of chlorogenic acids in the presence of amino acids leads to the formation of green benzacridines, which are a promising alternative to chlorophylls as food colorants.
中文翻译:

醌的反应-食品研究的机理,结构和前景
植物酚类化合物的氧化会生成醌,醌是可能与亲核试剂反应的不稳定中间体。醌在水果和蔬菜的酶促褐变中起重要作用,并可能与氨基酸,肽和蛋白质形成共价加合物。这些反应可能会改变食物蛋白质的物理化学和免疫学性质。醌会捕获有气味的化合物,并通过氨基酸的Strecker降解促进形成香气化合物。在氨基酸存在下,绿原酸的氧化二聚导致绿色苯甲cr啶的形成,这是作为食用色素替代叶绿素的一种有前途的替代方法。
更新日期:2018-11-25
中文翻译:

醌的反应-食品研究的机理,结构和前景
植物酚类化合物的氧化会生成醌,醌是可能与亲核试剂反应的不稳定中间体。醌在水果和蔬菜的酶促褐变中起重要作用,并可能与氨基酸,肽和蛋白质形成共价加合物。这些反应可能会改变食物蛋白质的物理化学和免疫学性质。醌会捕获有气味的化合物,并通过氨基酸的Strecker降解促进形成香气化合物。在氨基酸存在下,绿原酸的氧化二聚导致绿色苯甲cr啶的形成,这是作为食用色素替代叶绿素的一种有前途的替代方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号