当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nano‐Cu‐Mediated Multi‐Site Approach to Ultrafine MoO2 Nanoparticles on Poly(diallyldimethylammonium chloride)‐Decorated Reduced Graphene Oxide for Hydrogen Evolution Electrocatalysis
ChemSusChem ( IF 7.5 ) Pub Date : 2019-01-07 , DOI: 10.1002/cssc.201802491 Min Tian 1 , Feng Li 1 , Haiguo Hu 1 , Jiantai Ma 1 , Jun Jin 1
ChemSusChem ( IF 7.5 ) Pub Date : 2019-01-07 , DOI: 10.1002/cssc.201802491 Min Tian 1 , Feng Li 1 , Haiguo Hu 1 , Jiantai Ma 1 , Jun Jin 1
Affiliation
|
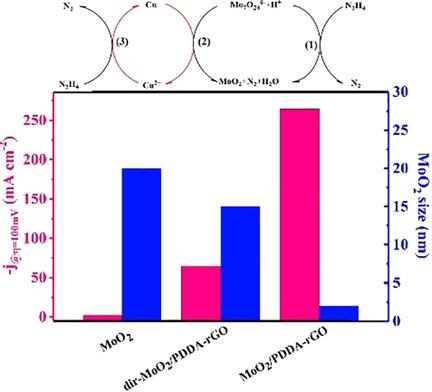
Catalysts with high atom utilization efficiency accompanied by improved reactivity and durability are highly desired. Metallic MoO2 with its small size easily agglomerates, making it difficult to use in water splitting to obtain hydrogen by electrolysis. Here, the nano‐Cu‐mediated multi‐site method is proposed to prepare ultrafine MoO2 nanoparticles (NPs) dispersed on poly(diallyldimethylammonium chloride)‐decorated reduced graphene oxide (denoted as MoO2/PDDA‐rGO). The introduction of Cu NPs increases the number of growth sites for MoO2 on the PDDA‐rGO and simultaneously promotes the growth rate of MoO2 on PDDA‐rGO. As a consequence, the resulting size of the MoO2 NPs is only 2 nm and these are evenly dispersed on PDDA‐rGO. Significantly, the optimized catalyst has a low onset potential of −42 mV versus reversible hydrogen electrode (RHE), a calculated Tafel slope of only 42 mV dec−1, and good cycling stability of more than 40 h. This favored hydrogen evolution reaction (HER) activity is caused by the synergistic effects of MoO2 and PDDA‐rGO, rapid charge transport, and sufficient exposed active sites of MoO2/PDDA‐rGO.
中文翻译:

纳米铜介导的多站点方法在聚二烯丙基二甲基氯化铵装饰的还原氧化石墨烯上超细MoO2纳米粒子的析氢电催化作用
非常需要具有高原子利用效率并具有改善的反应性和耐久性的催化剂。尺寸小的金属MoO 2容易团聚,难以用于通过电解的水分解法制氢。在这里,提出了纳米铜介导的多位点方法,以制备分散在聚二烯丙基二甲基氯化铵装饰的还原氧化石墨烯(表示为MoO 2 / PDDA- rGO)上的超细MoO 2纳米颗粒(NP )。Cu NPs的引入增加了PDDA-rGO上MoO 2的生长位点数量,同时提高了PDDA-rGO上MoO 2的生长速率。结果,MoO 2的最终尺寸NP仅2 nm,并且均匀分散在PDDA-rGO上。值得注意的是,与可逆氢电极(RHE)相比,优化后的催化剂具有-42 mV的低启动电位,计算出的Tafel斜率仅为42 mV dec -1,并且具有超过40 h的良好循环稳定性。MoO 2和PDDA-rGO的协同效应,快速的电荷传输以及MoO 2 / PDDA-rGO的足够暴露的活性位点引起了这种氢气放出反应(HER)活性的升高。
更新日期:2019-01-07
中文翻译:

纳米铜介导的多站点方法在聚二烯丙基二甲基氯化铵装饰的还原氧化石墨烯上超细MoO2纳米粒子的析氢电催化作用
非常需要具有高原子利用效率并具有改善的反应性和耐久性的催化剂。尺寸小的金属MoO 2容易团聚,难以用于通过电解的水分解法制氢。在这里,提出了纳米铜介导的多位点方法,以制备分散在聚二烯丙基二甲基氯化铵装饰的还原氧化石墨烯(表示为MoO 2 / PDDA- rGO)上的超细MoO 2纳米颗粒(NP )。Cu NPs的引入增加了PDDA-rGO上MoO 2的生长位点数量,同时提高了PDDA-rGO上MoO 2的生长速率。结果,MoO 2的最终尺寸NP仅2 nm,并且均匀分散在PDDA-rGO上。值得注意的是,与可逆氢电极(RHE)相比,优化后的催化剂具有-42 mV的低启动电位,计算出的Tafel斜率仅为42 mV dec -1,并且具有超过40 h的良好循环稳定性。MoO 2和PDDA-rGO的协同效应,快速的电荷传输以及MoO 2 / PDDA-rGO的足够暴露的活性位点引起了这种氢气放出反应(HER)活性的升高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号