Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
TIC236 links the outer and inner membrane translocons of the chloroplast
Nature ( IF 50.5 ) Pub Date : 2018-11-21 , DOI: 10.1038/s41586-018-0713-y Yih-Lin Chen , Lih-Jen Chen , Chiung-Chih Chu , Po-Kai Huang , Jie-Ru Wen , Hsou-min Li
Nature ( IF 50.5 ) Pub Date : 2018-11-21 , DOI: 10.1038/s41586-018-0713-y Yih-Lin Chen , Lih-Jen Chen , Chiung-Chih Chu , Po-Kai Huang , Jie-Ru Wen , Hsou-min Li
|
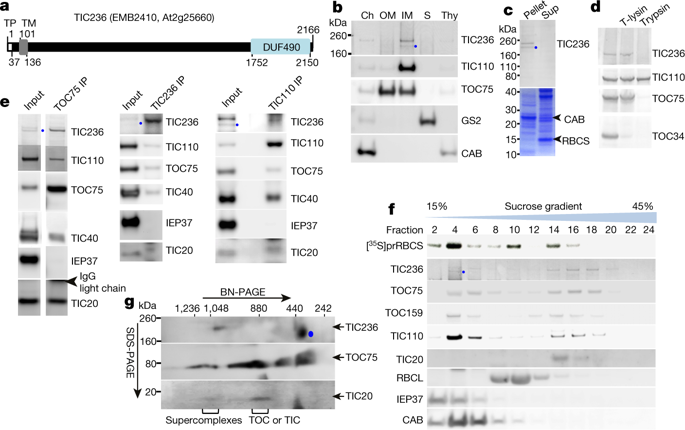
The two-membrane envelope is a defining feature of chloroplasts. Chloroplasts evolved from a Gram-negative cyanobacterial endosymbiont. During evolution, genes of the endosymbiont have been transferred to the host nuclear genome. Most chloroplast proteins are synthesized in the cytosol as higher-molecular-mass preproteins with an N-terminal transit peptide. Preproteins are transported into chloroplasts by the TOC and TIC (translocons at the outer- and inner-envelope membranes of chloroplasts, respectively) machineries1,2, but how TOC and TIC are assembled together is unknown. Here we report the identification of the TIC component TIC236; TIC236 is an integral inner-membrane protein that projects a 230-kDa domain into the intermembrane space, which binds directly to the outer-membrane channel TOC75. The knockout mutation of TIC236 is embryonically lethal. In TIC236-knockdown mutants, a smaller amount of the inner-membrane channel TIC20 was associated with TOC75; the amount of TOC–TIC supercomplexes was also reduced. This resulted in a reduced import rate into the stroma, though outer-membrane protein insertion was unaffected. The size and the essential nature of TIC236 indicate that—unlike in mitochondria, in which the outer- and inner-membrane translocons exist as separate complexes and a supercomplex is only transiently assembled during preprotein translocation3,4—a long and stable protein bridge in the intermembrane space is required for protein translocation into chloroplasts. Furthermore, TIC236 and TOC75 are homologues of bacterial inner-membrane TamB5 and outer-membrane BamA, respectively. Our evolutionary analyses show that, similar to TOC75, TIC236 is preserved only in plants and has co-evolved with TOC75 throughout the plant lineage. This suggests that the backbone of the chloroplast protein-import machinery evolved from the bacterial TamB–BamA protein-secretion system.TIC236 is an inner-membrane protein that binds to the outer-membrane channel TOC75 to create a long, stable bridge that enables the transport of proteins into chloroplasts.
中文翻译:

TIC236 连接叶绿体的外膜和内膜易位子
双膜包膜是叶绿体的一个决定性特征。叶绿体由革兰氏阴性蓝细菌内共生体进化而来。在进化过程中,内共生体的基因已转移到宿主核基因组中。大多数叶绿体蛋白在胞质溶胶中合成为具有 N 末端转运肽的较高分子量前蛋白。前蛋白通过 TOC 和 TIC(分别位于叶绿体外膜和内膜的易位子)机制 1,2 转运到叶绿体中,但 TOC 和 TIC 如何组装在一起尚不清楚。在此,我们报告了TIC成分TIC236的鉴定; TIC236 是一种完整的内膜蛋白,它将 230 kDa 的结构域投射到膜间隙中,直接与外膜通道 TOC75 结合。 TIC236 的敲除突变是胚胎致死的。在 TIC236 敲低突变体中,较少量的内膜通道 TIC20 与 TOC75 相关; TOC-TIC 超级复合物的量也减少了。这导致基质输入率降低,但外膜蛋白插入不受影响。 TIC236 的大小和本质表明,与线粒体不同,线粒体中的外膜和内膜易位子作为单独的复合物存在,而超级复合物仅在前蛋白易位过程中短暂组装3,4——线粒体中存在长且稳定的蛋白桥。蛋白质易位到叶绿体中需要膜间空间。此外,TIC236和TOC75分别是细菌内膜TamB5和外膜BamA的同源物。我们的进化分析表明,与 TOC75 类似,TIC236 仅保留在植物中,并且在整个植物谱系中与 TOC75 共同进化。 这表明叶绿体蛋白输入机制的主干是从细菌 TamB-BamA 蛋白分泌系统进化而来的。TIC236 是一种内膜蛋白,它与外膜通道 TOC75 结合,形成一个长而稳定的桥,使将蛋白质运输到叶绿体中。
更新日期:2018-11-21
中文翻译:

TIC236 连接叶绿体的外膜和内膜易位子
双膜包膜是叶绿体的一个决定性特征。叶绿体由革兰氏阴性蓝细菌内共生体进化而来。在进化过程中,内共生体的基因已转移到宿主核基因组中。大多数叶绿体蛋白在胞质溶胶中合成为具有 N 末端转运肽的较高分子量前蛋白。前蛋白通过 TOC 和 TIC(分别位于叶绿体外膜和内膜的易位子)机制 1,2 转运到叶绿体中,但 TOC 和 TIC 如何组装在一起尚不清楚。在此,我们报告了TIC成分TIC236的鉴定; TIC236 是一种完整的内膜蛋白,它将 230 kDa 的结构域投射到膜间隙中,直接与外膜通道 TOC75 结合。 TIC236 的敲除突变是胚胎致死的。在 TIC236 敲低突变体中,较少量的内膜通道 TIC20 与 TOC75 相关; TOC-TIC 超级复合物的量也减少了。这导致基质输入率降低,但外膜蛋白插入不受影响。 TIC236 的大小和本质表明,与线粒体不同,线粒体中的外膜和内膜易位子作为单独的复合物存在,而超级复合物仅在前蛋白易位过程中短暂组装3,4——线粒体中存在长且稳定的蛋白桥。蛋白质易位到叶绿体中需要膜间空间。此外,TIC236和TOC75分别是细菌内膜TamB5和外膜BamA的同源物。我们的进化分析表明,与 TOC75 类似,TIC236 仅保留在植物中,并且在整个植物谱系中与 TOC75 共同进化。 这表明叶绿体蛋白输入机制的主干是从细菌 TamB-BamA 蛋白分泌系统进化而来的。TIC236 是一种内膜蛋白,它与外膜通道 TOC75 结合,形成一个长而稳定的桥,使将蛋白质运输到叶绿体中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号