Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release
Nature ( IF 50.5 ) Pub Date : 2018-11-01 , DOI: 10.1038/s41586-018-0735-5 Sho Morioka 1, 2 , Justin S A Perry 1, 2 , Michael H Raymond 1, 3 , Christopher B Medina 1, 2 , Yunlu Zhu 4 , Liyang Zhao 5 , Vlad Serbulea 6 , Suna Onengut-Gumuscu 7 , Norbert Leitinger 6 , Sarah Kucenas 4 , Jeffrey C Rathmell 8 , Liza Makowski 5, 9 , Kodi S Ravichandran 1, 2, 10
Nature ( IF 50.5 ) Pub Date : 2018-11-01 , DOI: 10.1038/s41586-018-0735-5 Sho Morioka 1, 2 , Justin S A Perry 1, 2 , Michael H Raymond 1, 3 , Christopher B Medina 1, 2 , Yunlu Zhu 4 , Liyang Zhao 5 , Vlad Serbulea 6 , Suna Onengut-Gumuscu 7 , Norbert Leitinger 6 , Sarah Kucenas 4 , Jeffrey C Rathmell 8 , Liza Makowski 5, 9 , Kodi S Ravichandran 1, 2, 10
Affiliation
|
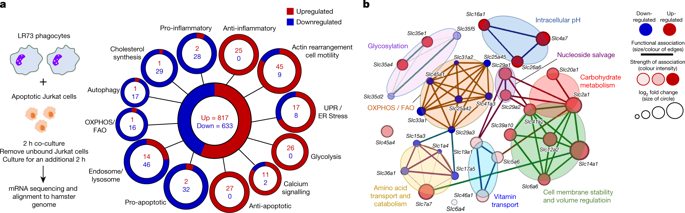
Development and routine tissue homeostasis require a high turnover of apoptotic cells. These cells are removed by professional and non-professional phagocytes via efferocytosis1. How a phagocyte maintains its homeostasis while coordinating corpse uptake, processing ingested materials and secreting anti-inflammatory mediators is incompletely understood1,2. Here, using RNA sequencing to characterize the transcriptional program of phagocytes actively engulfing apoptotic cells, we identify a genetic signature involving 33 members of the solute carrier (SLC) family of membrane transport proteins, in which expression is specifically modulated during efferocytosis, but not during antibody-mediated phagocytosis. We assessed the functional relevance of these SLCs in efferocytic phagocytes and observed a robust induction of an aerobic glycolysis program, initiated by SLC2A1-mediated glucose uptake, with concurrent suppression of the oxidative phosphorylation program. The different steps of phagocytosis2—that is, ‘smell’ (‘find-me’ signals or sensing factors released by apoptotic cells), ‘taste’ (phagocyte–apoptotic cell contact) and ‘ingestion’ (corpse internalization)—activated distinct and overlapping sets of genes, including several SLC genes, to promote glycolysis. SLC16A1 was upregulated after corpse uptake, increasing the release of lactate, a natural by-product of aerobic glycolysis3. Whereas glycolysis within phagocytes contributed to actin polymerization and the continued uptake of corpses, lactate released via SLC16A1 promoted the establishment of an anti-inflammatory tissue environment. Collectively, these data reveal a SLC program that is activated during efferocytosis, identify a previously unknown reliance on aerobic glycolysis during apoptotic cell uptake and show that glycolytic by-products of efferocytosis can influence surrounding cells.Distinct transcriptional programs are activated during different stages of apoptotic cell engulfment, including a unique program of genes coding for solute carrier proteins and enzymes in the glycolytic pathway.
中文翻译:

Efferocytosis 诱导新的 SLC 程序以促进葡萄糖摄取和乳酸释放
发育和常规组织稳态需要凋亡细胞的高周转。这些细胞通过 efferocytosis 被专业和非专业吞噬细胞去除。吞噬细胞如何在协调尸体摄取、处理摄入的材料和分泌抗炎介质的同时维持其体内平衡尚不完全清楚1,2。在这里,使用 RNA 测序来表征吞噬细胞主动吞噬凋亡细胞的转录程序,我们确定了一个涉及溶质载体 (SLC) 膜转运蛋白家族的 33 个成员的遗传特征,其中表达在胞吞作用过程中受到特异性调节,但在胞吞作用过程中不受调控。抗体介导的吞噬作用。我们评估了这些 SLC 在 efferocytic 吞噬细胞中的功能相关性,并观察到有氧糖酵解程序的强烈诱导,由 SLC2A1 介导的葡萄糖摄取引发,同时抑制氧化磷酸化程序。吞噬作用 2 的不同步骤——即“嗅觉”(凋亡细胞释放的“找到我”信号或传感因子)、“味觉”(吞噬细胞-凋亡细胞接触)和“摄入”(尸体内化)——激活了不同的和重叠的基因组,包括几个 SLC 基因,以促进糖酵解。SLC16A1 在尸体摄取后上调,增加乳酸的释放,乳酸是有氧糖酵解的天然副产物 3。虽然吞噬细胞内的糖酵解有助于肌动蛋白聚合和尸体的持续吸收,但通过 SLC16A1 释放的乳酸促进了抗炎组织环境的建立。总的来说,这些数据揭示了在胞吞作用过程中激活的 SLC 程序,
更新日期:2018-11-01
中文翻译:

Efferocytosis 诱导新的 SLC 程序以促进葡萄糖摄取和乳酸释放
发育和常规组织稳态需要凋亡细胞的高周转。这些细胞通过 efferocytosis 被专业和非专业吞噬细胞去除。吞噬细胞如何在协调尸体摄取、处理摄入的材料和分泌抗炎介质的同时维持其体内平衡尚不完全清楚1,2。在这里,使用 RNA 测序来表征吞噬细胞主动吞噬凋亡细胞的转录程序,我们确定了一个涉及溶质载体 (SLC) 膜转运蛋白家族的 33 个成员的遗传特征,其中表达在胞吞作用过程中受到特异性调节,但在胞吞作用过程中不受调控。抗体介导的吞噬作用。我们评估了这些 SLC 在 efferocytic 吞噬细胞中的功能相关性,并观察到有氧糖酵解程序的强烈诱导,由 SLC2A1 介导的葡萄糖摄取引发,同时抑制氧化磷酸化程序。吞噬作用 2 的不同步骤——即“嗅觉”(凋亡细胞释放的“找到我”信号或传感因子)、“味觉”(吞噬细胞-凋亡细胞接触)和“摄入”(尸体内化)——激活了不同的和重叠的基因组,包括几个 SLC 基因,以促进糖酵解。SLC16A1 在尸体摄取后上调,增加乳酸的释放,乳酸是有氧糖酵解的天然副产物 3。虽然吞噬细胞内的糖酵解有助于肌动蛋白聚合和尸体的持续吸收,但通过 SLC16A1 释放的乳酸促进了抗炎组织环境的建立。总的来说,这些数据揭示了在胞吞作用过程中激活的 SLC 程序,











































 京公网安备 11010802027423号
京公网安备 11010802027423号