当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A versatile strategy for the synthesis of sequence-defined peptoids with side-chain and backbone diversity via amino acid building blocks†
Chemical Science ( IF 7.6 ) Pub Date : 2018-11-22 00:00:00 , DOI: 10.1039/c8sc03415j Shixue Wang 1, 2 , Yue Tao 1, 3 , Jianqun Wang 1, 3 , Youhua Tao 1, 3 , Xianhong Wang 1, 3
Chemical Science ( IF 7.6 ) Pub Date : 2018-11-22 00:00:00 , DOI: 10.1039/c8sc03415j Shixue Wang 1, 2 , Yue Tao 1, 3 , Jianqun Wang 1, 3 , Youhua Tao 1, 3 , Xianhong Wang 1, 3
Affiliation
|
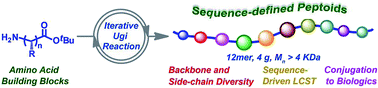
Designing artificial macromolecules with absolute sequence order is still a long-term challenge in polymer chemistry as opposed to natural biopolymers with perfectly defined sequences like proteins and DNA. Herein, we combined amino acid building blocks and iterative Ugi reactions for the de novo design and synthesis of sequence-defined peptoids. The highly efficient strategy provided excellent yields and enables multigram-scale synthesis of perfectly defined peptoids. This new strategy furnishes the broad structural diversity of side chains, as well as backbones. Importantly, the overall hydrophobicity and lower critical solution temperature (LCST) behaviours of these precisely defined peptoids can be logically altered by variation of the sequence. By following the same Ugi chemistry, these peptoids are also conjugated to DNA in a simple way, facilitating the development of novel therapeutics.
中文翻译:

通过氨基酸构建模块合成具有侧链和主链多样性的序列定义类肽的通用策略†
与蛋白质和DNA等具有完美定义序列的天然生物聚合物相比,设计具有绝对序列顺序的人造大分子仍然是高分子化学领域的长期挑战。在此,我们结合氨基酸构建模块和迭代 Ugi 反应来从头设计和合成序列定义的类肽。这种高效的策略提供了优异的产量,并能够实现精确定义的类肽的多克规模合成。这种新策略提供了侧链和主链的广泛结构多样性。重要的是,这些精确定义的类肽的整体疏水性和较低临界溶解温度(LCST)行为可以通过序列的变化而逻辑上改变。通过遵循相同的 Ugi 化学,这些类肽也以简单的方式与 DNA 缀合,促进了新型疗法的开发。
更新日期:2018-11-22
中文翻译:

通过氨基酸构建模块合成具有侧链和主链多样性的序列定义类肽的通用策略†
与蛋白质和DNA等具有完美定义序列的天然生物聚合物相比,设计具有绝对序列顺序的人造大分子仍然是高分子化学领域的长期挑战。在此,我们结合氨基酸构建模块和迭代 Ugi 反应来从头设计和合成序列定义的类肽。这种高效的策略提供了优异的产量,并能够实现精确定义的类肽的多克规模合成。这种新策略提供了侧链和主链的广泛结构多样性。重要的是,这些精确定义的类肽的整体疏水性和较低临界溶解温度(LCST)行为可以通过序列的变化而逻辑上改变。通过遵循相同的 Ugi 化学,这些类肽也以简单的方式与 DNA 缀合,促进了新型疗法的开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号