当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlling the speciation and reactivity of carbon-supported gold nanostructures for catalysed acetylene hydrochlorination†
Chemical Science ( IF 7.6 ) Pub Date : 2018-11-22 00:00:00 , DOI: 10.1039/c8sc03186j Selina K Kaiser 1 , Ronghe Lin 1 , Sharon Mitchell 1 , Edvin Fako 2 , Frank Krumeich 1 , Roland Hauert 3 , Olga V Safonova 4 , Vita A Kondratenko 5 , Evgenii V Kondratenko 5 , Sean M Collins 6 , Paul A Midgley 6 , Núria López 2 , Javier Pérez-Ramírez 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-11-22 00:00:00 , DOI: 10.1039/c8sc03186j Selina K Kaiser 1 , Ronghe Lin 1 , Sharon Mitchell 1 , Edvin Fako 2 , Frank Krumeich 1 , Roland Hauert 3 , Olga V Safonova 4 , Vita A Kondratenko 5 , Evgenii V Kondratenko 5 , Sean M Collins 6 , Paul A Midgley 6 , Núria López 2 , Javier Pérez-Ramírez 1
Affiliation
|
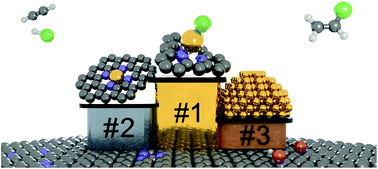
Carbon-supported gold catalysts have the potential to replace the toxic mercuric chloride-based system applied industrially for acetylene hydrochlorination, a key technology for the manufacture of polyvinyl chloride. However, the design of an optimal catalyst is essentially hindered by the difficulties in assessing the nature of the active site. Herein, we present a platform of carbon supported gold nanostructures at a fixed metal loading, ranging from single atoms of tunable oxidation state and coordination to metallic nanoparticles, by varying the structure of functionalised carbons and use of thermal activation. While on activated carbon particle aggregation occurs progressively above 473 K, on nitrogen-doped carbon gold single atoms exhibit outstanding stability up to temperatures of 1073 K and under reaction conditions. By combining steady-state experiments, density functional theory, and transient mechanistic studies, we assess the relation between the metal speciation, electronic properties, and catalytic activity. The results indicate that the activity of gold-based catalysts correlates with the population of Au(I)Cl single atoms and the reaction follows a Langmuir–Hinshelwood mechanism. Strong interaction with HCl and thermodynamically favoured acetylene activation were identified as the key features of the Au(I)Cl sites that endow their superior catalytic performance in comparison to N-stabilised Au(III) counterparts and gold nanoparticles. Finally, we show that the carrier (activated carbon versus nitrogen-doped carbon) does not affect the catalytic response, but determines the deactivation mechanism (gold particle aggregation and pore blockage, respectively), which opens up different options for the development of stable, high-performance hydrochlorination catalysts.
中文翻译:

控制催化乙炔氢氯化反应中碳载金纳米结构的形态和反应性†
碳载金催化剂有潜力取代工业上用于乙炔氢氯化的有毒氯化汞基系统,乙炔氢氯化是聚氯乙烯制造的关键技术。然而,最佳催化剂的设计本质上受到评估活性位点性质的困难的阻碍。在此,我们通过改变功能化碳的结构和使用热活化,提出了一个固定金属负载量的碳支撑金纳米结构平台,范围从可调氧化态和配位的单原子到金属纳米颗粒。在活性炭上,颗粒聚集在 473 K 以上逐渐发生,而在氮掺杂碳上,金单原子在高达 1073 K 的温度和反应条件下表现出出色的稳定性。通过结合稳态实验、密度泛函理论和瞬态机理研究,我们评估了金属形态、电子性质和催化活性之间的关系。结果表明,金基催化剂的活性与 Au()Cl 单原子的数量相关,反应遵循 Langmuir-Hinshelwood 机制。与 HCl 的强相互作用和热力学上有利的乙炔活化被认为是 Au()Cl 位点的关键特征,与 N 稳定的 Au( III ) 对应物和金纳米颗粒相比,它们赋予了其优越的催化性能。 最后,我们表明载体(活性炭与氮掺杂碳)不会影响催化响应,但决定失活机制(分别是金颗粒聚集和孔堵塞),这为开发稳定的、高性能氢氯化催化剂。
更新日期:2018-11-22
中文翻译:

控制催化乙炔氢氯化反应中碳载金纳米结构的形态和反应性†
碳载金催化剂有潜力取代工业上用于乙炔氢氯化的有毒氯化汞基系统,乙炔氢氯化是聚氯乙烯制造的关键技术。然而,最佳催化剂的设计本质上受到评估活性位点性质的困难的阻碍。在此,我们通过改变功能化碳的结构和使用热活化,提出了一个固定金属负载量的碳支撑金纳米结构平台,范围从可调氧化态和配位的单原子到金属纳米颗粒。在活性炭上,颗粒聚集在 473 K 以上逐渐发生,而在氮掺杂碳上,金单原子在高达 1073 K 的温度和反应条件下表现出出色的稳定性。通过结合稳态实验、密度泛函理论和瞬态机理研究,我们评估了金属形态、电子性质和催化活性之间的关系。结果表明,金基催化剂的活性与 Au()Cl 单原子的数量相关,反应遵循 Langmuir-Hinshelwood 机制。与 HCl 的强相互作用和热力学上有利的乙炔活化被认为是 Au()Cl 位点的关键特征,与 N 稳定的 Au( III ) 对应物和金纳米颗粒相比,它们赋予了其优越的催化性能。 最后,我们表明载体(活性炭与氮掺杂碳)不会影响催化响应,但决定失活机制(分别是金颗粒聚集和孔堵塞),这为开发稳定的、高性能氢氯化催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号