当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure–Activity Relationships of Hexahydrocyclopenta[c]quinoline Derivatives as Allosteric Inhibitors of CDK2 and EGFR
ChemMedChem ( IF 3.6 ) Pub Date : 2018-11-20 , DOI: 10.1002/cmdc.201800687 Luca Carlino 1 , Michael S. Christodoulou 1, 2 , Valentina Restelli 3 , Fabiana Caporuscio 1 , Francesca Foschi 1, 2 , Marta S. Semrau 4 , Elisa Costanzi 5 , Annachiara Tinivella 1 , Luca Pinzi 1 , Leonardo Lo Presti 2, 6 , Roberto Battistutta 5 , Paola Storici 4 , Massimo Broggini 3 , Daniele Passarella 2 , Giulio Rastelli 1
ChemMedChem ( IF 3.6 ) Pub Date : 2018-11-20 , DOI: 10.1002/cmdc.201800687 Luca Carlino 1 , Michael S. Christodoulou 1, 2 , Valentina Restelli 3 , Fabiana Caporuscio 1 , Francesca Foschi 1, 2 , Marta S. Semrau 4 , Elisa Costanzi 5 , Annachiara Tinivella 1 , Luca Pinzi 1 , Leonardo Lo Presti 2, 6 , Roberto Battistutta 5 , Paola Storici 4 , Massimo Broggini 3 , Daniele Passarella 2 , Giulio Rastelli 1
Affiliation
|
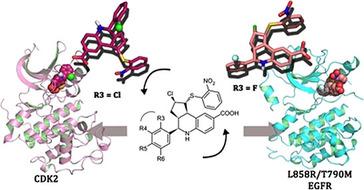
Following the discovery of a type III allosteric modulator of cyclin‐dependent kinase 2 (CDK2) characterized by a hexahydrocyclopenta[c]quinolone scaffold, three different series of its derivatives were synthesized and biologically evaluated. Docking of the synthesized compounds into the allosteric pocket of CDK2 allowed the elucidation of structure–activity relationships (SARs). Moreover, the compounds were tested on the wild‐type epidermal growth factor receptor (EGFR) kinase domain (KD) and its clinically relevant T790M/L858R mutant form. Herein we describe the first SAR investigation of allosteric ligands that bind to the type III inhibitor pocket of CDK2 and EGFR‐KD. Although the activity of the synthesized inhibitors needs to be improved, the obtained results provide clear‐cut indications about pharmacophore requirements and selectivity determinants. Remarkably, this study led to the identification of a selective T790M/L858R EGFR allosteric inhibitor that is inactive toward both wild‐type EGFR and CDK2. Finally, docking into the T790M/L858R EGFR‐KD led us to hypothesize that the compounds bind to the double‐mutant EGFR‐KD by adopting a binding mode different from that in CDK2, thus rationalizing the observed selectivity profile.
中文翻译:

六氢环戊五[c]喹啉衍生物作为CDK2和EGFR的变构抑制剂的构效关系。
在发现以六氢环戊五烯为特征的细胞周期蛋白依赖性激酶2(CDK2)的III型变构调节剂[ c喹诺酮支架,合成了三个不同系列的衍生物,并对其进行了生物学评估。将合成的化合物对接到CDK2的变构口袋中,可以阐明结构-活性关系(SAR)。此外,还对这些化合物进行了野生型表皮生长因子受体(EGFR)激酶域(KD)及其临床相关T790M / L858R突变体形式的测试。本文中,我们描述了与CDK2和EGFR-KD的III型抑制剂口袋结合的变构配体的首次SAR研究。尽管需要提高合成抑制剂的活性,但获得的结果提供了有关药效基团要求和选择性决定因素的明确指示。值得注意的是 这项研究导致鉴定出对野生型EGFR和CDK2均无活性的选择性T790M / L858R EGFR变构抑制剂。最后,对接至T790M / L858R EGFR-KD使我们假设化合物通过采用不同于CDK2的结合模式与双突变型EGFR-KD结合,从而使观察到的选择性分布合理化。
更新日期:2018-11-20
中文翻译:

六氢环戊五[c]喹啉衍生物作为CDK2和EGFR的变构抑制剂的构效关系。
在发现以六氢环戊五烯为特征的细胞周期蛋白依赖性激酶2(CDK2)的III型变构调节剂[ c喹诺酮支架,合成了三个不同系列的衍生物,并对其进行了生物学评估。将合成的化合物对接到CDK2的变构口袋中,可以阐明结构-活性关系(SAR)。此外,还对这些化合物进行了野生型表皮生长因子受体(EGFR)激酶域(KD)及其临床相关T790M / L858R突变体形式的测试。本文中,我们描述了与CDK2和EGFR-KD的III型抑制剂口袋结合的变构配体的首次SAR研究。尽管需要提高合成抑制剂的活性,但获得的结果提供了有关药效基团要求和选择性决定因素的明确指示。值得注意的是 这项研究导致鉴定出对野生型EGFR和CDK2均无活性的选择性T790M / L858R EGFR变构抑制剂。最后,对接至T790M / L858R EGFR-KD使我们假设化合物通过采用不同于CDK2的结合模式与双突变型EGFR-KD结合,从而使观察到的选择性分布合理化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号