Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
VCAM-1+ macrophages guide the homing of HSPCs to a vascular niche
Nature ( IF 50.5 ) Pub Date : 2018-11-19 , DOI: 10.1038/s41586-018-0709-7 Dantong Li , Wenzhi Xue , Mei Li , Mei Dong , Jianwei Wang , Xianda Wang , Xiyue Li , Kai Chen , Wenjuan Zhang , Shuang Wu , Yingqi Zhang , Lei Gao , Yujie Chen , Jianfeng Chen , Bo O. Zhou , Yi Zhou , Xuebiao Yao , Lin Li , Dianqing Wu , Weijun Pan
Nature ( IF 50.5 ) Pub Date : 2018-11-19 , DOI: 10.1038/s41586-018-0709-7 Dantong Li , Wenzhi Xue , Mei Li , Mei Dong , Jianwei Wang , Xianda Wang , Xiyue Li , Kai Chen , Wenjuan Zhang , Shuang Wu , Yingqi Zhang , Lei Gao , Yujie Chen , Jianfeng Chen , Bo O. Zhou , Yi Zhou , Xuebiao Yao , Lin Li , Dianqing Wu , Weijun Pan
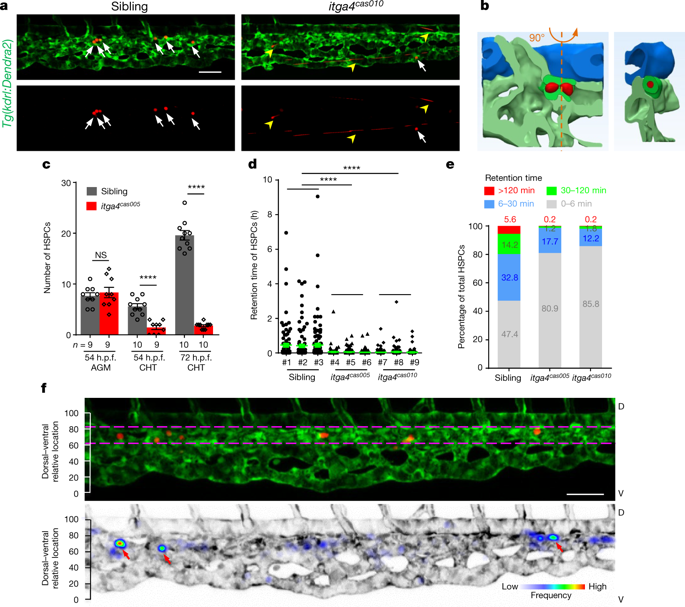
|
Haematopoietic stem and progenitor cells (HSPCs) give rise to all blood lineages that support the entire lifespan of vertebrates1. After HSPCs emerge from endothelial cells within the developing dorsal aorta, homing allows the nascent cells to anchor in their niches for further expansion and differentiation2–5. Unique niche microenvironments, composed of various blood vessels as units of microcirculation and other niche components such as stromal cells, regulate this process6–9. However, the detailed architecture of the microenvironment and the mechanism for the regulation of HSPC homing remain unclear. Here, using advanced live imaging and a cell-labelling system, we perform high-resolution analyses of the HSPC homing in caudal haematopoietic tissue of zebrafish (equivalent to the fetal liver in mammals), and reveal the role of the vascular architecture in the regulation of HSPC retention. We identify a VCAM-1+ macrophage-like niche cell population that patrols the inner surface of the venous plexus, interacts with HSPCs in an ITGA4-dependent manner, and directs HSPC retention. These cells, named ‘usher cells’, together with caudal venous capillaries and plexus, define retention hotspots within the homing microenvironment. Thus, the study provides insights into the mechanism of HSPC homing and reveals the essential role of a VCAM-1+ macrophage population with patrolling behaviour in HSPC retention.In zebrafish embryogenesis, nascent haematopoietic stem and progenitor cells (HSPCs), homing to a vascular niche for retention, are ushered by patrolling and guiding macrophages through integrin-mediated cell-cell recognition.
中文翻译:

VCAM-1+ 巨噬细胞引导 HSPC 归巢到血管壁龛
造血干细胞和祖细胞 (HSPC) 产生支持脊椎动物整个生命周期的所有血液谱系。在发育中的背主动脉内的内皮细胞中出现 HSPC 后,归巢允许新生细胞锚定在它们的壁龛中以进一步扩增和分化 2-5。由作为微循环单位的各种血管和基质细胞等其他生态位成分组成的独特生态位微环境调节这一过程 6-9。然而,微环境的详细结构和调控 HSPC 归巢的机制仍不清楚。在这里,我们使用先进的实时成像和细胞标记系统,对斑马鱼尾部造血组织(相当于哺乳动物的胎肝)中的 HSPC 进行高分辨率分析,并揭示血管结构在调节 HSPC 保留中的作用。我们确定了一个 VCAM-1+ 巨噬细胞样生态位细胞群,它在静脉丛内表面巡逻,以 ITGA4 依赖性方式与 HSPC 相互作用,并指导 HSPC 保留。这些细胞,称为“引导细胞”,连同尾静脉毛细血管和神经丛,定义了归巢微环境中的滞留热点。因此,该研究提供了对 HSPC 归巢机制的见解,并揭示了具有巡逻行为的 VCAM-1+ 巨噬细胞群在 HSPC 保留中的重要作用。在斑马鱼胚胎发生中,新生造血干细胞和祖细胞 (HSPC),归巢至血管通过整合素介导的细胞 - 细胞识别来巡逻和引导巨噬细胞。
更新日期:2018-11-19
中文翻译:

VCAM-1+ 巨噬细胞引导 HSPC 归巢到血管壁龛
造血干细胞和祖细胞 (HSPC) 产生支持脊椎动物整个生命周期的所有血液谱系。在发育中的背主动脉内的内皮细胞中出现 HSPC 后,归巢允许新生细胞锚定在它们的壁龛中以进一步扩增和分化 2-5。由作为微循环单位的各种血管和基质细胞等其他生态位成分组成的独特生态位微环境调节这一过程 6-9。然而,微环境的详细结构和调控 HSPC 归巢的机制仍不清楚。在这里,我们使用先进的实时成像和细胞标记系统,对斑马鱼尾部造血组织(相当于哺乳动物的胎肝)中的 HSPC 进行高分辨率分析,并揭示血管结构在调节 HSPC 保留中的作用。我们确定了一个 VCAM-1+ 巨噬细胞样生态位细胞群,它在静脉丛内表面巡逻,以 ITGA4 依赖性方式与 HSPC 相互作用,并指导 HSPC 保留。这些细胞,称为“引导细胞”,连同尾静脉毛细血管和神经丛,定义了归巢微环境中的滞留热点。因此,该研究提供了对 HSPC 归巢机制的见解,并揭示了具有巡逻行为的 VCAM-1+ 巨噬细胞群在 HSPC 保留中的重要作用。在斑马鱼胚胎发生中,新生造血干细胞和祖细胞 (HSPC),归巢至血管通过整合素介导的细胞 - 细胞识别来巡逻和引导巨噬细胞。











































 京公网安备 11010802027423号
京公网安备 11010802027423号