当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ionic Hydrogen and Halogen Bonding in the Gas Phase Association of Acetonitrile and Acetone with Halogenated Benzene Cations
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-11-19 00:00:00 , DOI: 10.1021/acs.jpca.8b09094 Adam C. Pearcy 1 , Kyle A. Mason 1 , M. Samy El-Shall 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-11-19 00:00:00 , DOI: 10.1021/acs.jpca.8b09094 Adam C. Pearcy 1 , Kyle A. Mason 1 , M. Samy El-Shall 1
Affiliation
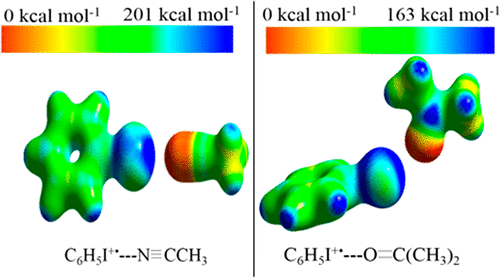
|
We report on the gas phase association of the small polar and aprotic solvent molecules acetonitrile (CH3CN) and acetone (CH3COCH3) with the halogenated benzene radical cations (C6H5X•+, X = F, Cl, Br, and I) using the mass-selected ion mobility technique and density functional theory calculations. The association energies (−ΔH°) of CH3CN (CH3COCH3) with C6H5F•+ and C6H5I•+ are similar [13.0 (13.3) and 13.2 (14.1) kcal/mol, respectively] but higher than those of CH3CN (CH3COCH3) with C6H5Cl•+ and C6H5Br•+ [10.5 (11.5) and 10.9 (10.6) kcal/mol, respectively]. However, the electrostatic potentials of the lowest energy structures of C6H5Br•+(CH3CN) and C6H5Br•+(CH3COCH3) or C6H5I•+(CH3CN) and C6H5I•+(CH3COCH3) complexes clearly show the formation of the ionic halogen bonds (IXBs) C–Brδ+- -NCCH3 and C–Brδ+- -OC(CH3)2 or C–Iδ+- -NCCH3 and C–Iδ+- -OC(CH3)2 driven by positively charged σ-holes on the external sides of the C–Br and C–I bond axes of the bromobenzene and iodobenzene radical cations, respectively. For the C6H5F•+(CH3CN) complex, the dominant interaction involves a T-shaped structure between the N atom of CH3CN and the C atom of the C–F bond of C6H5F•+. The structure of the C6H5Cl•+(CH3CN) complex shows the formation of unconventional ionic hydrogen bonds (uIHBs) between the N atom of CH3CN and the C–H bonds of the C6H5Cl•+ cation. Similar results are obtained for the association of acetone with the halogenated benzene radical cations. The formation of IXBs of the iodobenzene cation with acetonitrile or acetone involves a significant entropy loss (−ΔS° = 25–27 cal /(mol K)) resulting from the formation of more ordered and highly directional structures between the nitrogen or oxygen lone pair of electrons of acetonitrile or acetone, respectively, and the electropositive region around the iodine atom of the iodobenzene cation. In comparison, for the association of acetonitrile or acetone with the fluorobenzene, chlorobenzene, and bromobenzene cations, −ΔS° = 16–23 cal/(mol K), consistent with the formation of less ordered structures and loose interactions. The lowest energy structures of the C6H5Br•+(CH3COCH3)2 and C6H5I•+(CH3COCH3)2 clusters show a novel combination of ionic halogen bonding and hydrogen bonding where the oxygen atom of one acetone molecule forms the halogen bond while the oxygen atom of the second acetone molecule becomes the hydrogen acceptor from the methyl group of the first acetone molecule.
中文翻译:

乙腈和丙酮与卤代苯阳离子的气相缔合中的离子氢和卤素键合
我们报告了极性和非质子传递溶剂的小分子乙腈(CH 3 CN)和丙酮(CH 3 COCH 3)与卤代苯自由基阳离子(C 6 H 5 X •+,X = F,Cl, Br和I)使用质量选择离子迁移率技术和密度泛函理论计算。关联能量(-Δ ħ °)的CH 3 CN(CH 3 COCH 3)以C 6 H ^ 5 ˚F •+和C 6 H ^ 5我•+相似[分别为13.0(13.3)和13.2(14.1)kcal / mol],但高于具有C 6 H 5 Cl •+和C 6 H 5 Br •+的CH 3 CN(CH 3 COCH 3)[10.5 (11.5)和10.9(10.6)kcal / mol]。但是,C 6 H 5 Br •+(CH 3 CN)和C 6 H 5 Br •+(CH 3 COCH 3)或C 6 H 5 I •+的最低能量结构的静电势(CH 3 CN)和C 6 H 5 I •+(CH 3 COCH 3)配合物清楚地表明离子卤素键(IXBs)的形成是C– Brδ + --NCCH 3和C– Brδ + --OC (CH 3)2或C– Iδ + --NCCH 3和C– Iδ + --OC(CH 3)2受C–Br和C–I键外侧带正电的σ孔的驱动分别为溴苯和碘苯自由基的轴。对于C 6 H 5 F •+(CH3 CN)络合物,主要的相互作用涉及CH 3 CN的N原子与C 6 H 5 F •+的C–F键的C原子之间的T形结构。C 6 H 5 Cl •+(CH 3 CN)配合物的结构表明,CH 3 CN的N原子与C 6 H 5 Cl •的C–H键之间形成了非常规离子氢键(uIHBs)。+阳离子。对于丙酮与卤代苯自由基阳离子的缔合,获得了相似的结果。用乙腈或丙酮的碘苯阳离子的IXBs的形成包括一个显著熵损失(-Δ小号°= 25-27卡/(摩尔k))的从氮或氧之间孤形成更有序的和高度方向性的结构得到的乙腈或丙酮的一对电子,以及碘苯阳离子的碘原子周围的正电区域。相比之下,乙腈或丙酮的关联与氟苯,氯苯,溴苯和阳离子,-Δ小号°= 16-23卡/(摩尔K),以较少的有序结构和松动相互作用的形成是一致的。C的最低能量结构6 H 5 Br •+(CH 3 COCH 3)2和C 6 H 5 I •+(CH 3 COCH 3)2簇显示离子卤素键和氢键的新颖组合,其中一个丙酮分子的氧原子形成当第二个丙酮分子的氧原子成为第一个丙酮分子的甲基的氢受体时,卤素键。
更新日期:2018-11-19
中文翻译:

乙腈和丙酮与卤代苯阳离子的气相缔合中的离子氢和卤素键合
我们报告了极性和非质子传递溶剂的小分子乙腈(CH 3 CN)和丙酮(CH 3 COCH 3)与卤代苯自由基阳离子(C 6 H 5 X •+,X = F,Cl, Br和I)使用质量选择离子迁移率技术和密度泛函理论计算。关联能量(-Δ ħ °)的CH 3 CN(CH 3 COCH 3)以C 6 H ^ 5 ˚F •+和C 6 H ^ 5我•+相似[分别为13.0(13.3)和13.2(14.1)kcal / mol],但高于具有C 6 H 5 Cl •+和C 6 H 5 Br •+的CH 3 CN(CH 3 COCH 3)[10.5 (11.5)和10.9(10.6)kcal / mol]。但是,C 6 H 5 Br •+(CH 3 CN)和C 6 H 5 Br •+(CH 3 COCH 3)或C 6 H 5 I •+的最低能量结构的静电势(CH 3 CN)和C 6 H 5 I •+(CH 3 COCH 3)配合物清楚地表明离子卤素键(IXBs)的形成是C– Brδ + --NCCH 3和C– Brδ + --OC (CH 3)2或C– Iδ + --NCCH 3和C– Iδ + --OC(CH 3)2受C–Br和C–I键外侧带正电的σ孔的驱动分别为溴苯和碘苯自由基的轴。对于C 6 H 5 F •+(CH3 CN)络合物,主要的相互作用涉及CH 3 CN的N原子与C 6 H 5 F •+的C–F键的C原子之间的T形结构。C 6 H 5 Cl •+(CH 3 CN)配合物的结构表明,CH 3 CN的N原子与C 6 H 5 Cl •的C–H键之间形成了非常规离子氢键(uIHBs)。+阳离子。对于丙酮与卤代苯自由基阳离子的缔合,获得了相似的结果。用乙腈或丙酮的碘苯阳离子的IXBs的形成包括一个显著熵损失(-Δ小号°= 25-27卡/(摩尔k))的从氮或氧之间孤形成更有序的和高度方向性的结构得到的乙腈或丙酮的一对电子,以及碘苯阳离子的碘原子周围的正电区域。相比之下,乙腈或丙酮的关联与氟苯,氯苯,溴苯和阳离子,-Δ小号°= 16-23卡/(摩尔K),以较少的有序结构和松动相互作用的形成是一致的。C的最低能量结构6 H 5 Br •+(CH 3 COCH 3)2和C 6 H 5 I •+(CH 3 COCH 3)2簇显示离子卤素键和氢键的新颖组合,其中一个丙酮分子的氧原子形成当第二个丙酮分子的氧原子成为第一个丙酮分子的甲基的氢受体时,卤素键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号