当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Investigation of RO2 + HO2 and RO2 + RO2 Reactions of Monoterpene Derived First-Generation Peroxy Radicals Leading to Radical Recycling
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-11-19 00:00:00 , DOI: 10.1021/acs.jpca.8b09241 Siddharth Iyer 1 , Heidi Reiman 2 , Kristian H. Møller 3 , Matti P. Rissanen 4 , Henrik G. Kjaergaard 3 , Theo Kurtén 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-11-19 00:00:00 , DOI: 10.1021/acs.jpca.8b09241 Siddharth Iyer 1 , Heidi Reiman 2 , Kristian H. Møller 3 , Matti P. Rissanen 4 , Henrik G. Kjaergaard 3 , Theo Kurtén 1
Affiliation
|
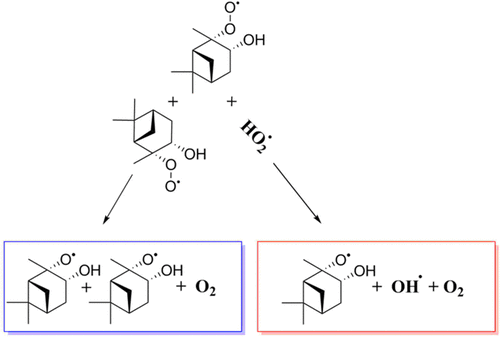
The oxidation of biogenically emitted volatile organic compounds (BVOC) plays an important role in the formation of secondary organic aerosols (SOA) in the atmosphere. Peroxy radicals (RO2) are central intermediates in the BVOC oxidation process. Under clean (low-NOx) conditions, the main bimolecular sink reactions for RO2 are with the hydroperoxy radical (HO2) and with other RO2 radicals. Especially for small RO2, the RO2 + HO2 reaction mainly leads to closed-shell hydroperoxide products. However, there exist other known RO2 + HO2 and RO2 + RO2 reaction channels that can recycle radicals and oxidants in the atmosphere, potentially leading to lower-volatility products and enhancing SOA formation. In this work, we present a thermodynamic overview of two such reactions: (a) RO2 + HO2 → RO + OH + O2 and (b) R′O2 + RO2 → R′O + RO + O2 for selected monoterpene + oxidant derived peroxy radicals. The monoterpenes considered are α-pinene, β-pinene, limonene, trans-β-ocimene, and Δ3-carene. The oxidants considered are the hydroxyl radical (OH), the nitrate radical (NO3), and ozone (O3). The reaction Gibbs energies were calculated at the DLPNO–CCSD(T)/def2-QZVPP//ωB97X-D/aug-cc-pVTZ level of theory. All reactions studied here were found to be exergonic in terms of Gibbs energy. On the basis of a comparison with previous mechanistic studies, we predict that reaction a and reaction b are likely to be most important for first-generation peroxy radicals from O3 oxidation (especially for β-pinene), while being less so for most first-generation peroxy radicals from OH and NO3 oxidation. This is because both reactions are comparatively more exergonic for the O3 oxidized systems than their OH and NO3 oxidized counterparts. Our results indicate that bimolecular reactions of certain complex RO2 may contribute to an increase in radical and oxidant recycling under high HO2 conditions in the atmosphere, which can potentially enhance SOA formation.
中文翻译:

单萜衍生的第一代过氧自由基导致自由基循环的RO 2 + HO 2和RO 2 + RO 2反应的计算研究
生物排放的挥发性有机化合物(BVOC)的氧化在大气中形成次级有机气溶胶(SOA)方面起着重要作用。过氧自由基(RO 2)是BVOC氧化过程中的主要中间体。下清洁(低NO X)的条件下,RO主双分子反应水槽2是与氢过自由基(HO 2),并与其他RO 2的基团。特别是对于小的RO 2,RO 2 + HO 2反应主要导致闭壳氢过氧化物产物。然而,存在其他已知的RO 2 + HO 2和RO 2 + RO2个反应通道,可以循环利用大气中的自由基和氧化剂,潜在地导致挥发性较低的产品并增强SOA的形成。在这项工作中,我们提出了两种这样的反应的热力学的概述:(1)RO 2 + HO 2 →RO + OH + O 2和(b)R'O 2 + RO 2 →R'O + RO + O 2为选择单萜+氧化剂衍生的过氧自由基。所考虑的是单萜α蒎烯,β蒎烯,苎烯,反式-β-罗勒烯,和Δ 3 -carene。所考虑的氧化剂是羟基(OH),硝酸根(NO 3)和臭氧(O 3)。吉布斯反应的能量是在理论上的DLPNO–CCSD(T)/ def2-QZVPP //ωB97X-D/ aug-cc-pVTZ的水平上计算的。根据吉布斯能量,发现这里研究的所有反应都是能动的。在与以前的机理研究进行比较的基础上,我们预测反应a和反应b对于从O 3氧化产生的第一代过氧自由基(尤其是对β-pine烯)而言可能是最重要的,而对于大多数第一个反应则不那么重要。 OH和NO 3氧化生成过氧自由基。这是因为相对于其OH和NO 3氧化的对应物,这两个反应对O 3氧化的体系都相对具有更大的能量。我们的结果表明某些复杂的RO 2的双分子反应可能会导致大气中高HO 2条件下自由基和氧化剂的循环利用增加,从而有可能增强SOA的形成。
更新日期:2018-11-19
中文翻译:

单萜衍生的第一代过氧自由基导致自由基循环的RO 2 + HO 2和RO 2 + RO 2反应的计算研究
生物排放的挥发性有机化合物(BVOC)的氧化在大气中形成次级有机气溶胶(SOA)方面起着重要作用。过氧自由基(RO 2)是BVOC氧化过程中的主要中间体。下清洁(低NO X)的条件下,RO主双分子反应水槽2是与氢过自由基(HO 2),并与其他RO 2的基团。特别是对于小的RO 2,RO 2 + HO 2反应主要导致闭壳氢过氧化物产物。然而,存在其他已知的RO 2 + HO 2和RO 2 + RO2个反应通道,可以循环利用大气中的自由基和氧化剂,潜在地导致挥发性较低的产品并增强SOA的形成。在这项工作中,我们提出了两种这样的反应的热力学的概述:(1)RO 2 + HO 2 →RO + OH + O 2和(b)R'O 2 + RO 2 →R'O + RO + O 2为选择单萜+氧化剂衍生的过氧自由基。所考虑的是单萜α蒎烯,β蒎烯,苎烯,反式-β-罗勒烯,和Δ 3 -carene。所考虑的氧化剂是羟基(OH),硝酸根(NO 3)和臭氧(O 3)。吉布斯反应的能量是在理论上的DLPNO–CCSD(T)/ def2-QZVPP //ωB97X-D/ aug-cc-pVTZ的水平上计算的。根据吉布斯能量,发现这里研究的所有反应都是能动的。在与以前的机理研究进行比较的基础上,我们预测反应a和反应b对于从O 3氧化产生的第一代过氧自由基(尤其是对β-pine烯)而言可能是最重要的,而对于大多数第一个反应则不那么重要。 OH和NO 3氧化生成过氧自由基。这是因为相对于其OH和NO 3氧化的对应物,这两个反应对O 3氧化的体系都相对具有更大的能量。我们的结果表明某些复杂的RO 2的双分子反应可能会导致大气中高HO 2条件下自由基和氧化剂的循环利用增加,从而有可能增强SOA的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号