当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, crystal structures, photoluminescence, magnetic and antioxidant properties, and theoretical analysis of Zn(ii) and Cu(ii) complexes of an aminoalcohol ligand supported by benzoate counter anions†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-11-19 00:00:00 , DOI: 10.1039/c8nj04122a Mantasha I. 1, 2, 3, 4 , M. Shahid 1, 2, 3, 4 , Musheer Ahmad 2, 3, 4, 5, 6 , Rahisuddin Rahisuddin 1, 4, 7 , Rizwan Arif 1, 4, 7 , Sana Tasneem 1, 2, 3, 4 , Farasha Sama 4, 8, 9 , Istikhar A. Ansari 4, 8, 9
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-11-19 00:00:00 , DOI: 10.1039/c8nj04122a Mantasha I. 1, 2, 3, 4 , M. Shahid 1, 2, 3, 4 , Musheer Ahmad 2, 3, 4, 5, 6 , Rahisuddin Rahisuddin 1, 4, 7 , Rizwan Arif 1, 4, 7 , Sana Tasneem 1, 2, 3, 4 , Farasha Sama 4, 8, 9 , Istikhar A. Ansari 4, 8, 9
Affiliation
|
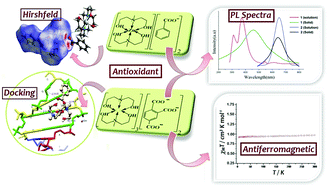
The coordination chemistry of two complexes [Zn(Me-deaH2)2][ba]2 (1) and [Cu(Me-deaH2)]3[tca]2 (2), where Me-deaH2 = N-methyldiethanolamine, Hba = benzoic acid and H3tca = 1,2,4-benzenetricarboxylic acid, has been explored. The Zn(II) and Cu(II) complexes are well characterized using spectral, single crystal X-ray and magnetic studies. The X-ray structure reveals a distorted octahedral geometry around both the metal ions. While the aminoalcoholate is coordinated with the metal ions, the benzoate moeity is present as a counter anion consolidating the crystal structure. Furthermore, the non-covalent interactions C–H⋯π, C–H⋯O, O–H⋯O and C–H⋯C result in 2D sheet networks in 1 and 2. These non-covalent interactions are theoretically predicted by Hirshfeld surface analysis. Solid state photoluminescence (PL) spectra of the complexes confirm their better luminescent behaviour than the corresponding parent conjugated ligands. The temperature variable magnetic data disclose the weak antiferromagnetic behaviour of 2. The plot indicates that the Cu(II) complex follows the Curie–Weiss law with a Curie constant, C = 0.29 cm3 K mol−1 and a Weiss constant, θ = −0.39 K. Moreover, the binding ability of both the complexes with DNA (PDB ID: 1BNA) is investigated theoretically and the data reveal the free energy of binding to be −256.22 (1) and −357.72 kcal mol−1 (2). The higher binding affinity of 2 can be rationalized in terms of its greater extent of H-bonding with DNA. Moreover, the assessment of the antioxidant properties employing DPPH free radical scavenging [IC50 = 0.628 ± 0.005 (1) and 0.850 ± 0.005 mg mL−1 (2)] and hydrogen peroxide assay [IC50 = 0.068 ± 0.001 (1) and 0.76 ± 0.01 mg mL−1 (2)] ascertains the Zn(II) complex to be a potent antioxidant.
中文翻译:

苯甲酸酯抗衡阴离子负载的氨基醇配体 的Zn(ii)和Cu(ii)配合物的合成,晶体结构,光致发光,磁性和抗氧化性质以及理论分析†
两种复合物[锌(ME-DEAH的配位化学2)2 ] [BA] 2(1)和[铜(ME-DEAH 2)] 3 [TCA] 2(2),其中Me-DEAH 2 = Ñ -已经研究了甲基二乙醇胺,Hba =苯甲酸,H 3 tca = 1,2,4-苯三甲酸。Zn(II)和Cu(II使用光谱,单晶X射线和磁性研究对复合物进行了很好的表征。X射线结构揭示了两种金属离子周围扭曲的八面体几何形状。氨基醇盐与金属离子配位时,苯甲酸酯部分作为反荷阴离子存在,巩固了晶体结构。此外,C–H⋯π,C–H⋯O,O–H⋯O和C–H⋯C的非共价相互作用导致1和2中的2D图纸网络。从理论上讲,这些非共价相互作用是通过Hirshfeld表面分析预测的。配合物的固态光致发光(PL)光谱证实其发光性能优于相应的母体共轭配体。温变磁数据揭示了2的弱反铁磁行为。该图表明Cu(II)配合物遵循居里-魏斯定律,居里常数C = 0.29 cm 3 K mol -1和魏斯常数θ = -0.39K。此外,两种配合物的结合能力从理论上研究了具有DNA(PDB ID:1BNA)的聚合物,数据显示结合的自由能为-256.22(1)和-357.72 kcal mol -1(2)。就其与DNA的H键结合程度而言,可以合理化2的较高结合亲和力。此外,使用DPPH自由基清除剂评估抗氧化性能[IC 50 = 0.628±0.005(1)和0.850±0.005 mg mL -1(2)]和过氧化氢测定[IC 50 = 0.068±0.001(1)和0.76±0.01 mg mL -1(2)]确定Zn(II)络合物为强效抗氧化剂。
更新日期:2018-11-19
中文翻译:

苯甲酸酯抗衡阴离子负载的氨基醇配体 的Zn(ii)和Cu(ii)配合物的合成,晶体结构,光致发光,磁性和抗氧化性质以及理论分析†
两种复合物[锌(ME-DEAH的配位化学2)2 ] [BA] 2(1)和[铜(ME-DEAH 2)] 3 [TCA] 2(2),其中Me-DEAH 2 = Ñ -已经研究了甲基二乙醇胺,Hba =苯甲酸,H 3 tca = 1,2,4-苯三甲酸。Zn(II)和Cu(II使用光谱,单晶X射线和磁性研究对复合物进行了很好的表征。X射线结构揭示了两种金属离子周围扭曲的八面体几何形状。氨基醇盐与金属离子配位时,苯甲酸酯部分作为反荷阴离子存在,巩固了晶体结构。此外,C–H⋯π,C–H⋯O,O–H⋯O和C–H⋯C的非共价相互作用导致1和2中的2D图纸网络。从理论上讲,这些非共价相互作用是通过Hirshfeld表面分析预测的。配合物的固态光致发光(PL)光谱证实其发光性能优于相应的母体共轭配体。温变磁数据揭示了2的弱反铁磁行为。该图表明Cu(II)配合物遵循居里-魏斯定律,居里常数C = 0.29 cm 3 K mol -1和魏斯常数θ = -0.39K。此外,两种配合物的结合能力从理论上研究了具有DNA(PDB ID:1BNA)的聚合物,数据显示结合的自由能为-256.22(1)和-357.72 kcal mol -1(2)。就其与DNA的H键结合程度而言,可以合理化2的较高结合亲和力。此外,使用DPPH自由基清除剂评估抗氧化性能[IC 50 = 0.628±0.005(1)和0.850±0.005 mg mL -1(2)]和过氧化氢测定[IC 50 = 0.068±0.001(1)和0.76±0.01 mg mL -1(2)]确定Zn(II)络合物为强效抗氧化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号