当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NiO-Modified Coconut Shell Based Activated Carbon Pretreated with KOH for the High-Efficiency Adsorption of NO at Ambient Temperature
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-12-03 , DOI: 10.1021/acs.iecr.8b03209 Fengyu Gao 1, 2 , Xiaolong Tang 1, 2 , Honghong Yi 1, 2 , Bowen Zhang 1 , Shunzheng Zhao 1, 2 , Jiangen Wang 1 , Tian Gu 1 , Yuhe Wang 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-12-03 , DOI: 10.1021/acs.iecr.8b03209 Fengyu Gao 1, 2 , Xiaolong Tang 1, 2 , Honghong Yi 1, 2 , Bowen Zhang 1 , Shunzheng Zhao 1, 2 , Jiangen Wang 1 , Tian Gu 1 , Yuhe Wang 1
Affiliation
|
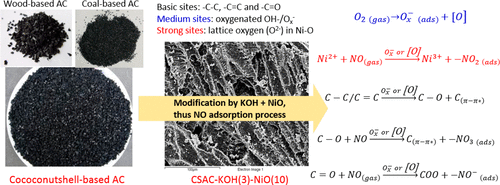
In this study, coconutshell-, coal-, and wood-based activated carbon were selected as the adsorbents for NO adsorption. KOH and transition metal oxides (CuO, FeOx, and NiO) were further adopted to improve the NO removal efficiency and adsorption capacity at ambient temperature. The results showed that CSAC-KOH(3)-NiO(10) adsorbents (coconut shell based activated carbon modified by 3% KOH and then 10% NiO) achieved the highest removal efficiency of NO (above 95.6%) and adsorption capacity (5.26 mg/g) within 60 min. XPS and NO-TPD results indicated that the increase of −C–O and OH–/Ox– species might be the reasons for good removal efficiency of NO over CSAC-KOH, while the increase of OH-/Ox– and lattice oxygen (O2– in Ni–O) species is the reason for the CSAC-KOH-NiO sample. The medium sites of the active adsorbed oxygen (Ox–) species and strong sites of lattice oxygen are the major adsorption sites on the surface of CSAC-KOH-NiO. The possible NO adsorption process were proposed that the main nitrite species (−NO2) might be generated by lattice oxygen (O2–) in Ni–O sites with the stimulation effects of active oxygen (Ox– or/and [O]), while NO– and – NO3 species were generated from −C—C, −C═C, and −C═O sites.
中文翻译:

KOH预处理的NiO改性椰子壳基活性炭在环境温度下高效吸附NO
在这项研究中,以椰子壳,煤炭和木材为基础的活性炭被选作NO吸附的吸附剂。进一步采用KOH和过渡金属氧化物(CuO,FeO x和NiO)来提高环境温度下的NO去除效率和吸附能力。结果表明,CSAC-KOH (3) -NiO (10)吸附剂(用3%KOH然后10%NiO改性的椰子壳基活性炭)实现了最高的NO去除效率(高于95.6%)和吸附能力(5.26)毫克/克)在60分钟内。XPS和NO-TPD结果表明,增加-C-O和OH- / O X -物种可能是NO的CSAC-KOH的去除效果良好的理由,而OH- / O的增加X -CSAC-KOH-NiO样品的原因是晶格中的氧(Ni-O中的O 2–)。活性吸附的氧的介质站点(O X - )物种和晶格氧的强位点是CSAC-KOH氧化镍的表面上的主要吸附位点。提出了可能的NO吸附过程,即主要的亚硝酸盐物种(-NO 2)可能是由Ni-O位点的晶格氧(O 2–)产生,并具有活性氧(O x –或/和[O]的刺激作用)。),而– C–C,–C═C和–C═O位产生了NO –和– NO 3物种。
更新日期:2018-12-03
中文翻译:

KOH预处理的NiO改性椰子壳基活性炭在环境温度下高效吸附NO
在这项研究中,以椰子壳,煤炭和木材为基础的活性炭被选作NO吸附的吸附剂。进一步采用KOH和过渡金属氧化物(CuO,FeO x和NiO)来提高环境温度下的NO去除效率和吸附能力。结果表明,CSAC-KOH (3) -NiO (10)吸附剂(用3%KOH然后10%NiO改性的椰子壳基活性炭)实现了最高的NO去除效率(高于95.6%)和吸附能力(5.26)毫克/克)在60分钟内。XPS和NO-TPD结果表明,增加-C-O和OH- / O X -物种可能是NO的CSAC-KOH的去除效果良好的理由,而OH- / O的增加X -CSAC-KOH-NiO样品的原因是晶格中的氧(Ni-O中的O 2–)。活性吸附的氧的介质站点(O X - )物种和晶格氧的强位点是CSAC-KOH氧化镍的表面上的主要吸附位点。提出了可能的NO吸附过程,即主要的亚硝酸盐物种(-NO 2)可能是由Ni-O位点的晶格氧(O 2–)产生,并具有活性氧(O x –或/和[O]的刺激作用)。),而– C–C,–C═C和–C═O位产生了NO –和– NO 3物种。











































 京公网安备 11010802027423号
京公网安备 11010802027423号