当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclic tris-[5]helicenes with single and triple twisted Möbius topologies and Möbius aromaticity†
Chemical Science ( IF 7.6 ) Pub Date : 2018-11-16 00:00:00 , DOI: 10.1039/c8sc02877j Guillaume Naulet 1 , Ludmilla Sturm 1 , Antoine Robert 1 , Pierre Dechambenoit 1 , Fynn Röhricht 2 , Rainer Herges 2 , Harald Bock 1 , Fabien Durola 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-11-16 00:00:00 , DOI: 10.1039/c8sc02877j Guillaume Naulet 1 , Ludmilla Sturm 1 , Antoine Robert 1 , Pierre Dechambenoit 1 , Fynn Röhricht 2 , Rainer Herges 2 , Harald Bock 1 , Fabien Durola 1
Affiliation
|
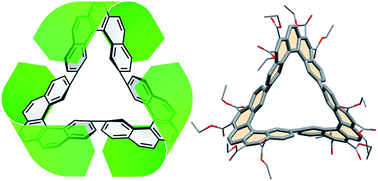
A number of singly (180°) twisted, largely single-stranded and thus conformationally rather fragile, Möbius molecules have been synthesized within the last 15 years, which are aromatic with 4n electrons, thus violating the Hückel rule. Annulenes with significantly higher twist (e.g. 540°) that retain a full cyclic conjugation path have been elusive, mainly because of the high strain and loss of orbital overlap. Recently, a topological strategy was devised to project the “twist” into “writhe”, thus reducing the strain. However, orbital overlap was still severely reduced within the flexible building blocks. We now present a single and a triple twisted annulene with fully conjugated peripheries. They are unique in their pronounced band shape and conformational robustness as they are made up of three fully kata-condensed [5]helicene fragments. The triple twisted molecule exhibits a strong diatropic ring current in the outer periphery, even though the π system includes 4n electrons. The diatropic current is counterbalanced by a paratropic current in the σ system, resulting in no net manifestation of macrocyclic aromaticity. The key step of the synthesis of both Möbius compounds is a Perkin condensation of complementary bifunctional bismaleates leading to a flexible macrocycle containing alternating benzene and biphenyl fragments. Subsequent photocyclization yields a separable mixture of rigid diastereomeric tris-helicene macrocycles of the above topologies.
中文翻译:

具有单扭曲和三扭曲莫比乌斯拓扑和莫比乌斯芳香性的环状三-[5]螺旋烯†
在过去 15 年里,人们合成了许多单 (180°) 扭曲、大部分为单链、因此构象相当脆弱的莫比乌斯分子,这些分子具有 4 n 个电子的芳香族结构,因此违反了休克尔规则。具有明显更高扭曲(例如540°)且保留完整循环共轭路径的轮烯一直难以捉摸,主要是因为高应变和轨道重叠的损失。最近,设计了一种拓扑策略,将“扭曲”投射为“扭动”,从而减少应变。然而,灵活构建块内的轨道重叠仍然严重减少。我们现在提出具有完全共轭外围的单扭曲轮烯和三扭曲轮烯。它们的独特之处在于其明显的带形状和构象稳健性,因为它们由三个完全缩合的[5]螺旋烯片段组成。尽管 π 系统包含 4 n 个电子,但三重扭曲分子在外周表现出很强的变异性环电流。σ 系统中的反异性电流被反异性电流抵消,导致大环芳香性没有净表现。两种莫比乌斯化合物合成的关键步骤是互补双功能双马来酸酯的 Perkin 缩合,形成包含交替苯和联苯片段的柔性大环。随后的光环化产生上述拓扑结构的刚性非对映体三螺旋烯大环的可分离混合物。
更新日期:2018-11-16
中文翻译:

具有单扭曲和三扭曲莫比乌斯拓扑和莫比乌斯芳香性的环状三-[5]螺旋烯†
在过去 15 年里,人们合成了许多单 (180°) 扭曲、大部分为单链、因此构象相当脆弱的莫比乌斯分子,这些分子具有 4 n 个电子的芳香族结构,因此违反了休克尔规则。具有明显更高扭曲(例如540°)且保留完整循环共轭路径的轮烯一直难以捉摸,主要是因为高应变和轨道重叠的损失。最近,设计了一种拓扑策略,将“扭曲”投射为“扭动”,从而减少应变。然而,灵活构建块内的轨道重叠仍然严重减少。我们现在提出具有完全共轭外围的单扭曲轮烯和三扭曲轮烯。它们的独特之处在于其明显的带形状和构象稳健性,因为它们由三个完全缩合的[5]螺旋烯片段组成。尽管 π 系统包含 4 n 个电子,但三重扭曲分子在外周表现出很强的变异性环电流。σ 系统中的反异性电流被反异性电流抵消,导致大环芳香性没有净表现。两种莫比乌斯化合物合成的关键步骤是互补双功能双马来酸酯的 Perkin 缩合,形成包含交替苯和联苯片段的柔性大环。随后的光环化产生上述拓扑结构的刚性非对映体三螺旋烯大环的可分离混合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号