当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkyl Sulfides as Promising Sulfur Sources: Metal‐Free Synthesis of Aryl Alkyl Sulfides and Dialkyl Sulfides by Transalkylation of Simple Sulfides with Alkyl Halides
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-11-27 , DOI: 10.1002/asia.201801679 Ting Liu 1 , Renhua Qiu 1, 2 , Longzhi Zhu 1 , Shuang-Feng Yin 1 , Chak-Tong Au 3 , Nobuaki Kambe 1, 2
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-11-27 , DOI: 10.1002/asia.201801679 Ting Liu 1 , Renhua Qiu 1, 2 , Longzhi Zhu 1 , Shuang-Feng Yin 1 , Chak-Tong Au 3 , Nobuaki Kambe 1, 2
Affiliation
|
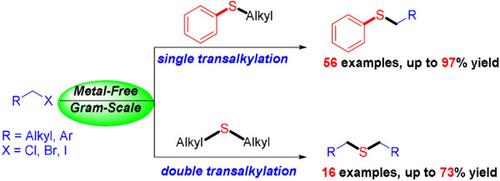
A site‐selective metal‐free dealkylative approach to synthesize aryl alkyl and symmetrical dialkyl sulfides has been developed. This procedure is convenient and has wide functional group tolerance giving rise to sulfides carrying various alkyl chains from simple alkyl sulfides and alkyl halides in good to excellent yields. This transalkylation proceeds by an ionic mechanism via sulfonium intermediates and it was proposed that dimethylacetamide (DMAC) may participate in part to promote the reaction.
中文翻译:

烷基硫化物作为有前景的硫来源:通过简单的硫化物与烷基卤化物的烷基转移,无金属合成芳基烷基硫化物和二烷基硫化物
已经开发出了一种位点选择的无金属脱烷基方法来合成芳基烷基和对称的二烷基硫化物。该方法是方便的并且具有宽的官能团耐受性,从而以良好的至优异的产率产生了从简单的烷基硫化物和烷基卤化物携带带有各种烷基链的硫化物。这种烷基转移反应是通过ionic中间体通过离子机理进行的,有人提出,二甲基乙酰胺(DMAC)可以部分参与促进反应。
更新日期:2018-11-27
中文翻译:

烷基硫化物作为有前景的硫来源:通过简单的硫化物与烷基卤化物的烷基转移,无金属合成芳基烷基硫化物和二烷基硫化物
已经开发出了一种位点选择的无金属脱烷基方法来合成芳基烷基和对称的二烷基硫化物。该方法是方便的并且具有宽的官能团耐受性,从而以良好的至优异的产率产生了从简单的烷基硫化物和烷基卤化物携带带有各种烷基链的硫化物。这种烷基转移反应是通过ionic中间体通过离子机理进行的,有人提出,二甲基乙酰胺(DMAC)可以部分参与促进反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号