当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Atmospheric Decomposition of Trifluoromethanol Catalyzed by Formic Acid
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-11-14 00:00:00 , DOI: 10.1021/acs.jpca.8b09316 Arathala Parandaman 1 , Josue E. Perez 1 , Amitabha Sinha 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-11-14 00:00:00 , DOI: 10.1021/acs.jpca.8b09316 Arathala Parandaman 1 , Josue E. Perez 1 , Amitabha Sinha 1
Affiliation
|
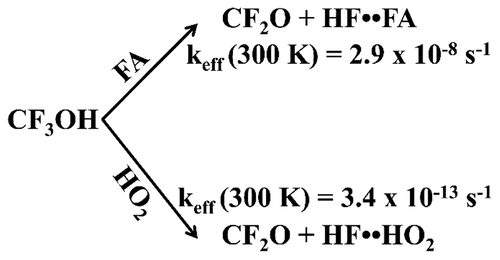
Quantum chemistry calculations are used to investigate the energetics and kinetics of CF3OH decomposition catalyzed by a single formic acid (FA) molecule acting alone and in conjunction with a single water (H2O) molecule to form the products carbonyl fluoride (CF2O) and hydrofluoric acid (HF). While the uncatalyzed reaction has a barrier of ∼44.7 kcal/mol, the presence of a FA molecule reduces the barrier to 6.4 kcal/mol, while the presence of both a FA and H2O molecule acting in unison decreases the barrier to −1.6 kcal/mol measured relative to the separated reactants. For comparison, we have also examined the decomposition of CF3OH catalyzed by HO2 and HO2 + H2O, which have been suggested in the literature to be an important atmospheric catalyst for CF3OH decomposition. In addition, we have also examined the loss of CF3OH via its bimolecular reaction with OH radicals. The rate constants for these various reactions were calculated using canonical variational transition state theory coupled with small curvature tunneling corrections over the temperature range between 200 and 300 K. Our results show that the rates for the CF3OH + FA and CF3OH + FA + H2O reactions are ∼104 times faster compared, respectively, to the corresponding reactions involving CF3OH + HO2 and CF3OH + HO2 + H2O at 300 K. Further, we find that, although the CF3OH + FA reaction has a higher barrier compared to CF3OH + FA + H2O, measured relative to the separated reagents, its effective first order rate for CF3OH decomposition is significantly faster for temperatures above 240 K compared to that of CF3OH + FA + H2O. This trend arises from the higher unimolecular reaction barrier for the reactant complex associated with the CF3OH + H2O + FA reaction compared to that for CF3OH + FA, as well as the lower concentration of reactant dimer complexes for CF3OH + H2O + FA compared to the concentration of the monomer FA reactant in the CF3OH + FA reaction. Finally, our calculations show that the rate for CF3OH decomposition catalyzed by FA is ∼104 times faster relative to the loss of CF3OH via its bimolecular reaction with OH radicals over the 200–300 K temperature range. Thus, the present study suggests that, among the various known loss mechanisms, a unimolecular reaction catalyzed by FA is likely the dominant gas phase decomposition pathway for CF3OH in the troposphere.
中文翻译:

甲酸催化三氟甲醇的常压分解
量子化学计算用于研究由单个甲酸(FA)分子单独作用并与单个水(H 2 O)分子结合形成碳酰氟(CF 2)产物催化的CF 3 OH分解的能量和动力学。O)和氢氟酸(HF)。尽管未催化反应的阻隔性约为44.7 kcal / mol,但FA分子的存在将其势垒降低至6.4 kcal / mol,而同时存在的FA和H 2 O分子将其势垒降低至-1.6相对于分离的反应物测量的kcal / mol。为了进行比较,我们还研究了HO 2和HO 2催化的CF 3 OH的分解+ H 2 O,在文献中已被认为是CF 3 OH分解的重要大气催化剂。此外,我们还研究了CF 3 OH通过与OH自由基的双分子反应而损失的情况。使用规范的变分过渡态理论以及200至300 K的温度范围内的小曲率隧穿校正,计算了这些反应的速率常数。我们的结果表明,CF 3 OH + FA和CF 3 OH + FA的速率+ H 2 O反应的速度分别比涉及CF 3 OH + HO 2的相应反应快约10 4倍和CF 3 OH + HO 2 + H 2 O在300 K时。此外,我们发现,尽管相对于分离的CF 3 OH + FA + H 2 O而言,CF 3 OH + FA反应具有更高的势垒与CF 3 OH + FA + H 2 O相比,在240 K以上的温度下,CF 3 OH分解的有效一阶速率明显更快。CF 3 OH + H 2 O + FA反应相比,对于CF 3与CF 3 OH + FA反应中单体FA反应物的浓度相比,CF 3 OH + H 2 O + FA中的OH + FA以及反应物二聚物配合物的浓度更低。最后,我们的计算表明,相对于CF 3 OH与200-300 K温度范围内与OH自由基的双分子反应,CF 3 OH分解CF 3 OH的速率快约10 4倍。因此,本研究表明,在各种已知的损失机制中,FA催化的单分子反应可能是对流层CF 3 OH的主要气相分解途径。
更新日期:2018-11-14
中文翻译:

甲酸催化三氟甲醇的常压分解
量子化学计算用于研究由单个甲酸(FA)分子单独作用并与单个水(H 2 O)分子结合形成碳酰氟(CF 2)产物催化的CF 3 OH分解的能量和动力学。O)和氢氟酸(HF)。尽管未催化反应的阻隔性约为44.7 kcal / mol,但FA分子的存在将其势垒降低至6.4 kcal / mol,而同时存在的FA和H 2 O分子将其势垒降低至-1.6相对于分离的反应物测量的kcal / mol。为了进行比较,我们还研究了HO 2和HO 2催化的CF 3 OH的分解+ H 2 O,在文献中已被认为是CF 3 OH分解的重要大气催化剂。此外,我们还研究了CF 3 OH通过与OH自由基的双分子反应而损失的情况。使用规范的变分过渡态理论以及200至300 K的温度范围内的小曲率隧穿校正,计算了这些反应的速率常数。我们的结果表明,CF 3 OH + FA和CF 3 OH + FA的速率+ H 2 O反应的速度分别比涉及CF 3 OH + HO 2的相应反应快约10 4倍和CF 3 OH + HO 2 + H 2 O在300 K时。此外,我们发现,尽管相对于分离的CF 3 OH + FA + H 2 O而言,CF 3 OH + FA反应具有更高的势垒与CF 3 OH + FA + H 2 O相比,在240 K以上的温度下,CF 3 OH分解的有效一阶速率明显更快。CF 3 OH + H 2 O + FA反应相比,对于CF 3与CF 3 OH + FA反应中单体FA反应物的浓度相比,CF 3 OH + H 2 O + FA中的OH + FA以及反应物二聚物配合物的浓度更低。最后,我们的计算表明,相对于CF 3 OH与200-300 K温度范围内与OH自由基的双分子反应,CF 3 OH分解CF 3 OH的速率快约10 4倍。因此,本研究表明,在各种已知的损失机制中,FA催化的单分子反应可能是对流层CF 3 OH的主要气相分解途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号