当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anodization of Bismuth: Measuring Breakdown Voltage and Optimizing an Electrolytic Cell
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-11-15 00:00:00 , DOI: 10.1021/acs.jchemed.8b00481 Thomas Nagel 1 , Casey Mentzer 1 , P. Mike Kivistik 2
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-11-15 00:00:00 , DOI: 10.1021/acs.jchemed.8b00481 Thomas Nagel 1 , Casey Mentzer 1 , P. Mike Kivistik 2
Affiliation
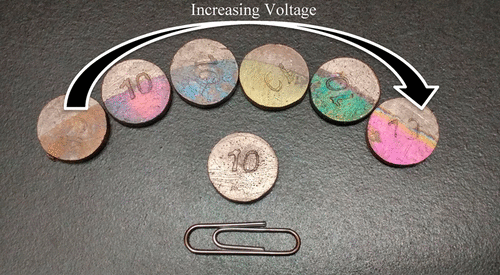
|
This experiment provides students with a thorough understanding of electrolysis and the parameters that most greatly impact optimization of this process. In this laboratory exercise, students will focus on the anodization of bismuth metal to develop thin oxide films. When designing an electrolytic cell devoted to anodization, there are several major factors necessary for the optimization of the cell and these are explored in this experiment. Current density, electrolyte concentration, breakdown voltage, and anodization rate all impact development of these anodic oxide films. This exercise emphasizes the interrelationships between these critical factors and the best way to optimize an electrolytic cell. This procedure is carried out with standard chemicals and widely available technology to allow for an inexpensive and accessible laboratory experiment.
中文翻译:

铋的阳极氧化:测量击穿电压并优化电解槽
该实验为学生提供了对电解的最全面的了解,以及对该过程的优化产生最大影响的参数。在此实验室练习中,学生将专注于铋金属的阳极氧化处理以形成薄氧化膜。在设计专门用于阳极氧化的电解池时,有几个主要因素需要优化电解池,在本实验中将对这些因素进行探讨。电流密度,电解质浓度,击穿电压和阳极氧化速率都影响这些阳极氧化膜的发展。本练习强调了这些关键因素与优化电解池的最佳方法之间的相互关系。
更新日期:2018-11-15
中文翻译:

铋的阳极氧化:测量击穿电压并优化电解槽
该实验为学生提供了对电解的最全面的了解,以及对该过程的优化产生最大影响的参数。在此实验室练习中,学生将专注于铋金属的阳极氧化处理以形成薄氧化膜。在设计专门用于阳极氧化的电解池时,有几个主要因素需要优化电解池,在本实验中将对这些因素进行探讨。电流密度,电解质浓度,击穿电压和阳极氧化速率都影响这些阳极氧化膜的发展。本练习强调了这些关键因素与优化电解池的最佳方法之间的相互关系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号