当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanisms of the Ethynyl Radical Reaction with Molecular Oxygen
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-11-13 00:00:00 , DOI: 10.1021/acs.jpca.8b09862 Michael C. Bowman 1 , Alexandra D. Burke 1 , Justin M. Turney 1 , Henry F. Schaefer 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-11-13 00:00:00 , DOI: 10.1021/acs.jpca.8b09862 Michael C. Bowman 1 , Alexandra D. Burke 1 , Justin M. Turney 1 , Henry F. Schaefer 1
Affiliation
|
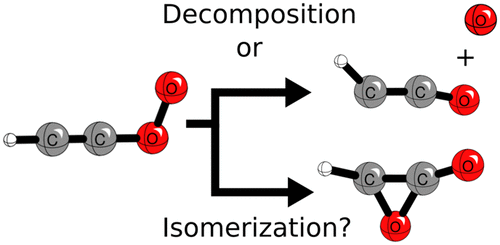
The ethynyl radical, •C2H, is a key intermediate in the combustion of various alkynes. Once produced, the ethynyl radical will rapidly react with molecular oxygen to produce a variety of products. This research presents the first comprehensive high level theoretical study of the reaction of the •C2H (2Σ+) radical with molecular oxygen (3Σg–). Correlation methods as complete as CCSDT(Q) were used; basis sets as large as cc-pV6Z were adopted. Focal point analysis was employed to approach relative energies within the bounds of chemical accuracy (≤1 kcal mol–1). Two dominate reaction pathways from the ethynyl peroxy radical include oxygen–oxygen cleavage from the ethynyl peroxy radical that is initially formed to produce HCCO (2A″) and O (3P) and an isomerization of the ethynyl peroxy radical to eventually yield HCO (2A′) and CO (1Σ+). The branching ratio between these two competitive reaction pathways was determined to be 1:1 at 298 K. Minor reaction pathways leading to the production of CO2 (1Σg+) and CH (2Π, 4Σ–, 2Δ) were also characterized. The absence of CCO (3Σ–) and OH (2Π) was explained in terms competition with more accessible reaction pathways.
中文翻译:

乙炔基与分子氧的自由基反应机理
乙炔基• C 2 H是各种炔烃燃烧的关键中间体。乙炔基一旦产生,将迅速与分子氧反应以产生多种产物。本研究呈现的反应的第一次全面高电平理论研究• Ç 2 H(2 Σ +)基团与分子氧(3 Σ克- )。使用了与CCSDT(Q)一样完整的相关方法。采用了与cc-pV6Z一样大的基集。使用焦点分析法来接近化学准确度范围内的相对能量(≤1kcal mol –1)。乙炔基过氧自由基的两个主要反应途径包括乙炔基过氧自由基的氧-氧裂解,最初形成该化合物以产生HCCO(2 A'')和O(3 P),以及乙炔基过氧自由基的异构化以最终产生HCO(2 A')和CO(1 Σ +)。确定这两个竞争反应途径之间的分支比为1:1在298K次要反应途径导致产生CO的2(1 Σ克+)和CH(2 Π,4 Σ - ,2 Δ)分别为也有特点。没有CCO(3 Σ - )和OH(2 Π)的混合物在术语竞争与更易接近的反应途径进行说明。
更新日期:2018-11-13
中文翻译:

乙炔基与分子氧的自由基反应机理
乙炔基• C 2 H是各种炔烃燃烧的关键中间体。乙炔基一旦产生,将迅速与分子氧反应以产生多种产物。本研究呈现的反应的第一次全面高电平理论研究• Ç 2 H(2 Σ +)基团与分子氧(3 Σ克- )。使用了与CCSDT(Q)一样完整的相关方法。采用了与cc-pV6Z一样大的基集。使用焦点分析法来接近化学准确度范围内的相对能量(≤1kcal mol –1)。乙炔基过氧自由基的两个主要反应途径包括乙炔基过氧自由基的氧-氧裂解,最初形成该化合物以产生HCCO(2 A'')和O(3 P),以及乙炔基过氧自由基的异构化以最终产生HCO(2 A')和CO(1 Σ +)。确定这两个竞争反应途径之间的分支比为1:1在298K次要反应途径导致产生CO的2(1 Σ克+)和CH(2 Π,4 Σ - ,2 Δ)分别为也有特点。没有CCO(3 Σ - )和OH(2 Π)的混合物在术语竞争与更易接近的反应途径进行说明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号