当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of a Key Intermediate for Tofacitinib via a Dynamic Kinetic Resolution-Reductive Amination Protocol
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2018-11-14 00:00:00 , DOI: 10.1021/acs.oprd.8b00332 Gerard K. M. Verzijl 1 , Christian Schuster 2 , Thomas Dax 2 , André H. M. de Vries 1 , Laurent Lefort 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2018-11-14 00:00:00 , DOI: 10.1021/acs.oprd.8b00332 Gerard K. M. Verzijl 1 , Christian Schuster 2 , Thomas Dax 2 , André H. M. de Vries 1 , Laurent Lefort 1
Affiliation
|
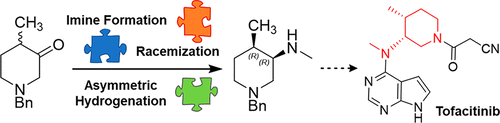
We report the first example of a catalytic asymmetric reductive amination under dynamic kinetic resolution (DKR) conditions for the preparation of a chiral amine as a key intermediate toward Tofacitinib, an active pharmaceutical ingredient developed by Pfizer. Such a protocol allows the preferential formation of a single product out of four possible diastereomers of the chiral amine starting from the corresponding racemic ketone. The chiral iridium catalyst able to perform such a feast was discovered through a mix of high-throughput screening, racemization study, and reaction optimization.
中文翻译:

托法替尼关键中间体的不对称合成,通过动态动力学拆分-还原胺化方案进行
我们报告了在动态动力学分辨率(DKR)条件下制备手性胺作为Tofacitinib(一种由辉瑞公司开发的活性药物成分的关键中间体)的关键中间体的催化不对称还原胺化的第一个例子。这样的方案允许从相应的外消旋酮开始,从四种可能的手性胺的非对映异构体中优先形成单个产物。通过高通量筛选,外消旋研究和反应优化相结合,发现了能够进行此类盛宴的手性铱催化剂。
更新日期:2018-11-14
中文翻译:

托法替尼关键中间体的不对称合成,通过动态动力学拆分-还原胺化方案进行
我们报告了在动态动力学分辨率(DKR)条件下制备手性胺作为Tofacitinib(一种由辉瑞公司开发的活性药物成分的关键中间体)的关键中间体的催化不对称还原胺化的第一个例子。这样的方案允许从相应的外消旋酮开始,从四种可能的手性胺的非对映异构体中优先形成单个产物。通过高通量筛选,外消旋研究和反应优化相结合,发现了能够进行此类盛宴的手性铱催化剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号