当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic and Electronic Polarization Corrections to the Dielectric Constant of Water
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-11-13 00:00:00 , DOI: 10.1021/acs.jpca.8b07953 Ardavan Farahvash 1 , Igor Leontyev 1 , Alexei Stuchebrukhov 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-11-13 00:00:00 , DOI: 10.1021/acs.jpca.8b07953 Ardavan Farahvash 1 , Igor Leontyev 1 , Alexei Stuchebrukhov 1
Affiliation
|
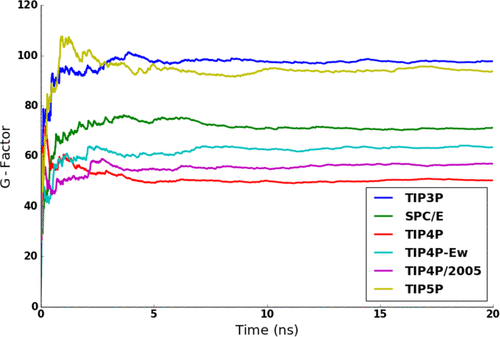
The standard approach to calculating the dielectric constant from molecular dynamics (MD) simulations employs a variant of the Kirkwood–Fröhlich methodology. Many popular nonpolarizable models of water, such as TIPnP, give a reasonable agreement with the experimental value of 78. However, it has been argued in the literature that the dipole moments of these models are effective, being smaller than the real dipole of a liquid water molecule by about a factor of  , or roughly
, or roughly  . If the total or corrected dipole moment is used in calculations, the dielectric constant comes out nearly twice as large, i.e., in the range of 160, which is twice as high as the experimental value. Here we discuss possible reasons for such a discrepancy. One approach takes into account dynamic corrections due to the dependence of the dielectric response of the medium producing the reaction field on the time scale of dipole fluctuations computed in the Kirkwood–Fröhlich method. When dynamic corrections are incorporated into the computational scheme, a much better agreement with the experimental value of the dielectric constant is found when the corrected (real) dipole moment of liquid water is used. However, a formal analysis indicates that the static properties, such as dielectric constant, should not depend on dynamics. We discuss the resulting conundrum and related issues of simulations of electrostatic interactions using periodic boundary conditions in the context of our findings.
. If the total or corrected dipole moment is used in calculations, the dielectric constant comes out nearly twice as large, i.e., in the range of 160, which is twice as high as the experimental value. Here we discuss possible reasons for such a discrepancy. One approach takes into account dynamic corrections due to the dependence of the dielectric response of the medium producing the reaction field on the time scale of dipole fluctuations computed in the Kirkwood–Fröhlich method. When dynamic corrections are incorporated into the computational scheme, a much better agreement with the experimental value of the dielectric constant is found when the corrected (real) dipole moment of liquid water is used. However, a formal analysis indicates that the static properties, such as dielectric constant, should not depend on dynamics. We discuss the resulting conundrum and related issues of simulations of electrostatic interactions using periodic boundary conditions in the context of our findings.
中文翻译:

水的介电常数的动态和电子极化校正
根据分子动力学(MD)模拟计算介电常数的标准方法采用了Kirkwood–Fröhlich方法的一种变体。许多流行的非极化水模型,例如TIPnP,与实验值78有着合理的一致性。但是,在文献中有人争辩说,这些模型的偶极矩有效,小于液体的真实偶极矩。水分子大约为 或的大约
或的大约 。如果在计算中使用了总偶极矩或校正后的偶极矩,则介电常数几乎是原来的两倍,即在160的范围内,是实验值的两倍。在这里,我们讨论了这种差异的可能原因。一种方法考虑到动态校正,这是由于产生反应场的介质的介电响应取决于在柯克伍德-弗洛里希方法中计算出的偶极子涨落的时间尺度。当将动态校正合并到计算方案中时,当使用校正后的(实际)液态水偶极矩时,可以找到与介电常数的实验值更好的一致性。但是,形式分析表明,静态特性(例如介电常数)不应依赖于动力学。
。如果在计算中使用了总偶极矩或校正后的偶极矩,则介电常数几乎是原来的两倍,即在160的范围内,是实验值的两倍。在这里,我们讨论了这种差异的可能原因。一种方法考虑到动态校正,这是由于产生反应场的介质的介电响应取决于在柯克伍德-弗洛里希方法中计算出的偶极子涨落的时间尺度。当将动态校正合并到计算方案中时,当使用校正后的(实际)液态水偶极矩时,可以找到与介电常数的实验值更好的一致性。但是,形式分析表明,静态特性(例如介电常数)不应依赖于动力学。
更新日期:2018-11-13
 , or roughly
, or roughly  . If the total or corrected dipole moment is used in calculations, the dielectric constant comes out nearly twice as large, i.e., in the range of 160, which is twice as high as the experimental value. Here we discuss possible reasons for such a discrepancy. One approach takes into account dynamic corrections due to the dependence of the dielectric response of the medium producing the reaction field on the time scale of dipole fluctuations computed in the Kirkwood–Fröhlich method. When dynamic corrections are incorporated into the computational scheme, a much better agreement with the experimental value of the dielectric constant is found when the corrected (real) dipole moment of liquid water is used. However, a formal analysis indicates that the static properties, such as dielectric constant, should not depend on dynamics. We discuss the resulting conundrum and related issues of simulations of electrostatic interactions using periodic boundary conditions in the context of our findings.
. If the total or corrected dipole moment is used in calculations, the dielectric constant comes out nearly twice as large, i.e., in the range of 160, which is twice as high as the experimental value. Here we discuss possible reasons for such a discrepancy. One approach takes into account dynamic corrections due to the dependence of the dielectric response of the medium producing the reaction field on the time scale of dipole fluctuations computed in the Kirkwood–Fröhlich method. When dynamic corrections are incorporated into the computational scheme, a much better agreement with the experimental value of the dielectric constant is found when the corrected (real) dipole moment of liquid water is used. However, a formal analysis indicates that the static properties, such as dielectric constant, should not depend on dynamics. We discuss the resulting conundrum and related issues of simulations of electrostatic interactions using periodic boundary conditions in the context of our findings.
中文翻译:

水的介电常数的动态和电子极化校正
根据分子动力学(MD)模拟计算介电常数的标准方法采用了Kirkwood–Fröhlich方法的一种变体。许多流行的非极化水模型,例如TIPnP,与实验值78有着合理的一致性。但是,在文献中有人争辩说,这些模型的偶极矩有效,小于液体的真实偶极矩。水分子大约为
 或的大约
或的大约 。如果在计算中使用了总偶极矩或校正后的偶极矩,则介电常数几乎是原来的两倍,即在160的范围内,是实验值的两倍。在这里,我们讨论了这种差异的可能原因。一种方法考虑到动态校正,这是由于产生反应场的介质的介电响应取决于在柯克伍德-弗洛里希方法中计算出的偶极子涨落的时间尺度。当将动态校正合并到计算方案中时,当使用校正后的(实际)液态水偶极矩时,可以找到与介电常数的实验值更好的一致性。但是,形式分析表明,静态特性(例如介电常数)不应依赖于动力学。
。如果在计算中使用了总偶极矩或校正后的偶极矩,则介电常数几乎是原来的两倍,即在160的范围内,是实验值的两倍。在这里,我们讨论了这种差异的可能原因。一种方法考虑到动态校正,这是由于产生反应场的介质的介电响应取决于在柯克伍德-弗洛里希方法中计算出的偶极子涨落的时间尺度。当将动态校正合并到计算方案中时,当使用校正后的(实际)液态水偶极矩时,可以找到与介电常数的实验值更好的一致性。但是,形式分析表明,静态特性(例如介电常数)不应依赖于动力学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号