Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Simultaneous Observation of Kinesin-Driven Microtubule Motility and Binding of Adenosine Triphosphate Using Linear Zero-Mode Waveguides
ACS Nano ( IF 15.8 ) Pub Date : 2018-11-12 00:00:00 , DOI: 10.1021/acsnano.8b03803 Kazuya Fujimoto 1 , Yuki Morita 1 , Ryota Iino 2 , Michio Tomishige 3 , Hirofumi Shintaku 1 , Hidetoshi Kotera 1 , Ryuji Yokokawa 1
ACS Nano ( IF 15.8 ) Pub Date : 2018-11-12 00:00:00 , DOI: 10.1021/acsnano.8b03803 Kazuya Fujimoto 1 , Yuki Morita 1 , Ryota Iino 2 , Michio Tomishige 3 , Hirofumi Shintaku 1 , Hidetoshi Kotera 1 , Ryuji Yokokawa 1
Affiliation
|
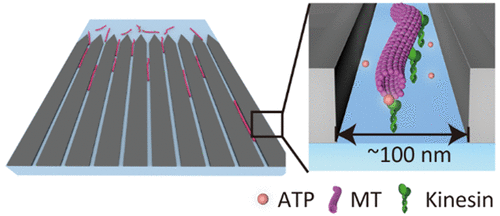
Single-molecule fluorescence observation of adenosine triphosphate (ATP) is a powerful tool to elucidate the chemomechanical coupling of ATP with a motor protein. However, in total internal reflection fluorescence microscopy (TIRFM), available ATP concentration is much lower than that in the in vivo environment. To achieve single-molecule observation with a high signal-to-noise ratio, zero-mode waveguides (ZMWs) are utilized even at high fluorescent molecule concentrations in the micromolar range. Despite the advantages of ZMWs, the use of cytoskeletal filaments for single-molecule observation has not been reported because of difficulties in immobilization of cytoskeletal filaments in the cylindrical aperture of ZMWs. Here, we propose linear ZMWs (LZMWs) to visualize enzymatic reactions on cytoskeletal filaments, specifically kinesin-driven microtubule motility accompanied by ATP binding/unbinding. Finite element method simulation revealed excitation light confinement in a 100 nm wide slit of LZMWs. Single-molecule observation was then demonstrated with up to 1 μM labeled ATP, which was 10-fold higher than that available in TIRFM. Direct observation of binding/unbinding of ATP to kinesins that propel microtubules enabled us to find that a significant fraction of ATP molecules bound to kinesins were dissociated without hydrolysis. This highlights the advantages of LZMWs for single-molecule observation of proteins that interact with cytoskeletal filaments such as microtubules, actin filaments, or intermediate filaments.
中文翻译:

使用线性零模式波导同时观察驱动蛋白驱动的微管运动和三磷酸腺苷的结合。
三磷酸腺苷(ATP)的单分子荧光观察是阐明ATP与运动蛋白的化学机械偶联的有力工具。但是,在全内反射荧光显微镜(TIRFM)中,可用的ATP浓度远低于体内的ATP浓度。环境。为了实现具有高信噪比的单分子观测,即使在微摩尔范围内的高荧光分子浓度下,也要使用零模式波导(ZMW)。尽管ZMW具有优势,但由于难以将细胞骨架细丝固定在ZMW圆柱孔中,因此尚未报道将细胞骨架细丝用于单分子观察。在这里,我们提出线性ZMWs(LZMWs)以可视化对细胞骨架细丝的酶促反应,特别是驱动蛋白驱动的微管运动并伴有ATP结合/解除结合。有限元方法仿真显示,激发光限制在LZMWs的100 nm宽缝中。然后证明了高达1μM标记的ATP的单分子观察,这是TIRFM中可用的10倍。直接观察ATP与驱动蛋白的结合/解除结合,从而推动微管,使我们发现与驱动蛋白结合的ATP分子中有很大一部分是在未水解的情况下解离的。这突出了LZMWs在单分子观察与细胞骨架细丝(例如微管,肌动蛋白细丝或中间细丝)相互作用的蛋白质时的优势。
更新日期:2018-11-12
中文翻译:

使用线性零模式波导同时观察驱动蛋白驱动的微管运动和三磷酸腺苷的结合。
三磷酸腺苷(ATP)的单分子荧光观察是阐明ATP与运动蛋白的化学机械偶联的有力工具。但是,在全内反射荧光显微镜(TIRFM)中,可用的ATP浓度远低于体内的ATP浓度。环境。为了实现具有高信噪比的单分子观测,即使在微摩尔范围内的高荧光分子浓度下,也要使用零模式波导(ZMW)。尽管ZMW具有优势,但由于难以将细胞骨架细丝固定在ZMW圆柱孔中,因此尚未报道将细胞骨架细丝用于单分子观察。在这里,我们提出线性ZMWs(LZMWs)以可视化对细胞骨架细丝的酶促反应,特别是驱动蛋白驱动的微管运动并伴有ATP结合/解除结合。有限元方法仿真显示,激发光限制在LZMWs的100 nm宽缝中。然后证明了高达1μM标记的ATP的单分子观察,这是TIRFM中可用的10倍。直接观察ATP与驱动蛋白的结合/解除结合,从而推动微管,使我们发现与驱动蛋白结合的ATP分子中有很大一部分是在未水解的情况下解离的。这突出了LZMWs在单分子观察与细胞骨架细丝(例如微管,肌动蛋白细丝或中间细丝)相互作用的蛋白质时的优势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号