当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and biological activities evaluation of sanjuanolide and its analogues.
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-11-10 , DOI: 10.1016/j.bmcl.2018.11.020 Jiadai Zhai 1 , Lin Fu 2 , Yuanyuan Li 2 , Rui Zhao 3 , Rui Wang 3 , Hongkuan Deng 2 , Hongliang Liu 2 , Ling Kong 2 , Zhiwei Chen 2 , Feng Sang 2
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-11-10 , DOI: 10.1016/j.bmcl.2018.11.020 Jiadai Zhai 1 , Lin Fu 2 , Yuanyuan Li 2 , Rui Zhao 3 , Rui Wang 3 , Hongkuan Deng 2 , Hongliang Liu 2 , Ling Kong 2 , Zhiwei Chen 2 , Feng Sang 2
Affiliation
|
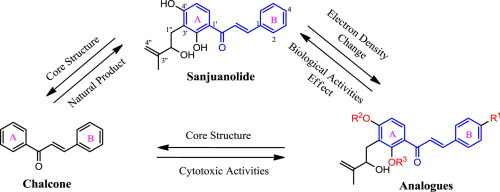
Sanjuanolide, psorachalcone A and its seven new analogues were synthesized via a combinatorial strategy by aldol reaction. In order to investigate the effect between electron density in π-conjugated systems and biological activities, several electron-withdrawing and electron-donating groups were introduced at C-4 and the phenolic hydroxyl groups of sanjuanolide. The two natural products and its seven new analogues were investigated for their inhibitory effects against five cancer cell lines. Moreover, the hydroxyisoprenyl group may be important to maintain the biological activities of sanjuanolide.
中文翻译:

Sanjuanolide及其类似物的合成及生物活性评估。
Sandolanolide,psorachalcone A及其七个新类似物是通过醛醇缩合反应通过组合策略合成的。为了研究π共轭体系中电子密度与生物活性之间的关系,在C-4处引入了几个吸电子基团和给电子基团,并引入了三juanolide的酚羟基。研究了这两种天然产物及其七个新类似物对五种癌细胞系的抑制作用。而且,羟基异戊二烯基对于维持三juanolide的生物活性可能是重要的。
更新日期:2018-11-10
中文翻译:

Sanjuanolide及其类似物的合成及生物活性评估。
Sandolanolide,psorachalcone A及其七个新类似物是通过醛醇缩合反应通过组合策略合成的。为了研究π共轭体系中电子密度与生物活性之间的关系,在C-4处引入了几个吸电子基团和给电子基团,并引入了三juanolide的酚羟基。研究了这两种天然产物及其七个新类似物对五种癌细胞系的抑制作用。而且,羟基异戊二烯基对于维持三juanolide的生物活性可能是重要的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号