当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiplex Assay for Quantification of Acute Phase Proteins and Immunoglobulin A in Dried Blood Spots
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-11-20 , DOI: 10.1021/acs.jproteome.8b00657 Veronika Vidova 1 , Eliska Stuchlikova 1 , Marketa Vrbova 1 , Martina Almasi 2 , Jana Klanova 1 , Vojtech Thon 1 , Zdenek Spacil 1
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-11-20 , DOI: 10.1021/acs.jproteome.8b00657 Veronika Vidova 1 , Eliska Stuchlikova 1 , Marketa Vrbova 1 , Martina Almasi 2 , Jana Klanova 1 , Vojtech Thon 1 , Zdenek Spacil 1
Affiliation
|
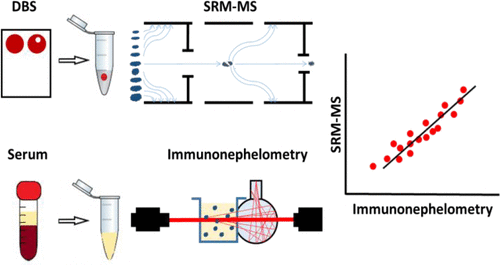
Inflammation is the first line defense mechanism against infection, tissue damage, or cancer development. However, inappropriate inflammatory response may also trigger diseases. The quantification of inflammatory proteins is essential to distinguish between harmful and beneficial immune response. Currently used immunoanalytical assays may suffer specificity issues due to antigen–antibody interaction and possible cross-reactivity of antibody with other protein species. In addition, immunoanalytical assays typically require invasive blood sampling and additional logistics; they are relatively costly and highly challenging to multiplex. We present a multiplex assay based on selected reaction monitoring (SRM) for quantification of seven acute-phase proteins (i.e., SAA1, SAA2-isoform1, SAA4, CRP, A1AT-isoform1, A1AG1, A1AG2) and the adaptive immunity effector IGHA1 in dried blood spots. This type of sample is readily available from all human subjects including newborns. The study utilizes proteotypic isotopically labeled peptides with trypsin-cleavable tag and presents optimized and reproducible workflow and several important practical remarks regarding quantitative SRM assays development. The panel of inflammatory proteins was quantified with sequence specificity capable to differentiate protein isoforms with intra- and interday precision (<16.4% coefficient of variation (CV) and <14.3% CV, respectively). Quantitative results were correlated with immuno-nephelometric assay (typically greater than 0.9 Pearson’s R).
中文翻译:

定量测定干血斑中急性期蛋白和免疫球蛋白A的多重分析
炎症是抵抗感染,组织损伤或癌症发展的一线防御机制。但是,不适当的炎症反应也可能引发疾病。炎症蛋白的定量对于区分有害和有益的免疫反应是必不可少的。由于抗原-抗体之间的相互作用以及抗体与其他蛋白质的交叉反应性,当前使用的免疫分析方法可能会遇到特异性问题。另外,免疫分析测定通常需要有创血液采样和额外的后勤服务。它们相对昂贵,并且复用非常困难。我们基于选定的反应监测(SRM)提出了一种定量测定七个急性期蛋白(即SAA1,SAA2-isoform1,SAA4,CRP,A1AT-isoform1,A1AG1,A1AG2)和干血斑中的自适应免疫效应子IGHA1。这种类型的样品可以从包括新生儿在内的所有人类受试者中容易地获得。这项研究利用具有胰蛋白酶可切割标签的蛋白同位素标记的肽,提出了优化和可重现的工作流程,以及有关定量SRM分析开发的一些重要实用说明。用能够以日内和日间精度(分别<16.4%变异系数(CV)和<14.3%CV)区分蛋白质同工型的序列特异性对炎性蛋白质组进行定量。定量结果与免疫比浊法相关(通常大于0.9 Pearson's 这项研究利用具有胰蛋白酶可切割标签的蛋白同位素标记的肽,提出了优化和可重现的工作流程,以及有关定量SRM分析开发的一些重要实用说明。用能够以日内和日间精度(分别<16.4%变异系数(CV)和<14.3%CV)区分蛋白质同工型的序列特异性对炎性蛋白质组进行定量。定量结果与免疫比浊法相关(通常大于0.9 Pearson's 这项研究利用具有胰蛋白酶可切割标签的蛋白同位素标记的肽,提出了优化和可重现的工作流程,以及有关定量SRM分析开发的一些重要实用说明。用能够以日内和日间精度(分别<16.4%变异系数(CV)和<14.3%CV)区分蛋白质同工型的序列特异性对炎性蛋白质组进行定量。定量结果与免疫比浊法相关(通常大于0.9 Pearson's 用能够以日内和日间精度(分别<16.4%变异系数(CV)和<14.3%CV)区分蛋白质同工型的序列特异性对炎性蛋白质组进行定量。定量结果与免疫比浊法相关(通常大于0.9 Pearson's 用能够以日内和日间精度(分别<16.4%变异系数(CV)和<14.3%CV)区分蛋白质同工型的序列特异性对炎性蛋白质组进行定量。定量结果与免疫比浊法相关(通常大于0.9 Pearson'sR)。
更新日期:2018-11-21
中文翻译:

定量测定干血斑中急性期蛋白和免疫球蛋白A的多重分析
炎症是抵抗感染,组织损伤或癌症发展的一线防御机制。但是,不适当的炎症反应也可能引发疾病。炎症蛋白的定量对于区分有害和有益的免疫反应是必不可少的。由于抗原-抗体之间的相互作用以及抗体与其他蛋白质的交叉反应性,当前使用的免疫分析方法可能会遇到特异性问题。另外,免疫分析测定通常需要有创血液采样和额外的后勤服务。它们相对昂贵,并且复用非常困难。我们基于选定的反应监测(SRM)提出了一种定量测定七个急性期蛋白(即SAA1,SAA2-isoform1,SAA4,CRP,A1AT-isoform1,A1AG1,A1AG2)和干血斑中的自适应免疫效应子IGHA1。这种类型的样品可以从包括新生儿在内的所有人类受试者中容易地获得。这项研究利用具有胰蛋白酶可切割标签的蛋白同位素标记的肽,提出了优化和可重现的工作流程,以及有关定量SRM分析开发的一些重要实用说明。用能够以日内和日间精度(分别<16.4%变异系数(CV)和<14.3%CV)区分蛋白质同工型的序列特异性对炎性蛋白质组进行定量。定量结果与免疫比浊法相关(通常大于0.9 Pearson's 这项研究利用具有胰蛋白酶可切割标签的蛋白同位素标记的肽,提出了优化和可重现的工作流程,以及有关定量SRM分析开发的一些重要实用说明。用能够以日内和日间精度(分别<16.4%变异系数(CV)和<14.3%CV)区分蛋白质同工型的序列特异性对炎性蛋白质组进行定量。定量结果与免疫比浊法相关(通常大于0.9 Pearson's 这项研究利用具有胰蛋白酶可切割标签的蛋白同位素标记的肽,提出了优化和可重现的工作流程,以及有关定量SRM分析开发的一些重要实用说明。用能够以日内和日间精度(分别<16.4%变异系数(CV)和<14.3%CV)区分蛋白质同工型的序列特异性对炎性蛋白质组进行定量。定量结果与免疫比浊法相关(通常大于0.9 Pearson's 用能够以日内和日间精度(分别<16.4%变异系数(CV)和<14.3%CV)区分蛋白质同工型的序列特异性对炎性蛋白质组进行定量。定量结果与免疫比浊法相关(通常大于0.9 Pearson's 用能够以日内和日间精度(分别<16.4%变异系数(CV)和<14.3%CV)区分蛋白质同工型的序列特异性对炎性蛋白质组进行定量。定量结果与免疫比浊法相关(通常大于0.9 Pearson'sR)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号