当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Loss of SETD2 Induces a Metabolic Switch in Renal Cell Carcinoma Cell Lines toward Enhanced Oxidative Phosphorylation
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-11-27 , DOI: 10.1021/acs.jproteome.8b00628 Jingping Liu 1, 2 , Paul D Hanavan 2 , Katon Kras 2 , Yvette W Ruiz 2 , Erik P Castle 3 , Douglas F Lake 2 , Xianfeng Chen 4 , Daniel O'Brien 5 , Huijun Luo 3 , Keith D Robertson 6 , Haiwei Gu 2 , Thai H Ho 7
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-11-27 , DOI: 10.1021/acs.jproteome.8b00628 Jingping Liu 1, 2 , Paul D Hanavan 2 , Katon Kras 2 , Yvette W Ruiz 2 , Erik P Castle 3 , Douglas F Lake 2 , Xianfeng Chen 4 , Daniel O'Brien 5 , Huijun Luo 3 , Keith D Robertson 6 , Haiwei Gu 2 , Thai H Ho 7
Affiliation
|
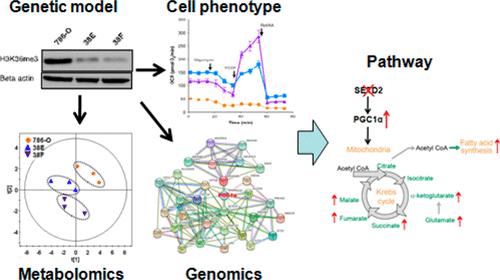
SETD2, a histone H3 lysine trimethyltransferase, is frequently inactivated and associated with recurrence of clear cell renal cell carcinoma (ccRCC). However, the impact of SETD2 loss on metabolic alterations in ccRCC is still unclear. In this study, SETD2 null isogenic 38E/38F clones derived from 786-O cells were generated by zinc finger nucleases, and subsequent metabolic, genomic, and cellular phenotypic changes were analyzed by targeted metabolomics, RNA sequencing, and biological methods, respectively. Our results showed that compared with parental 786-O cells, 38E/38F cells had elevated levels of MTT/Alamar blue levels, ATP, glycolytic/mitochondrial respiratory capacity, citrate synthase (CS) activity, and TCA metabolites such as aspartate, malate, succinate, fumarate, and α-ketoglutarate. The 38E/38F cells also utilized alternative sources beyond pyruvate to generate acetyl-CoA for the TCA cycle. Moreover, 38E/38F cells showed disturbed gene networks mainly related to mitochondrial metabolism and the oxidation of fatty acids and glucose, which was associated with increased PGC1α, mitochondrial mass, and cellular size/complexity. Our results indicate that SETD2 deficiency induces a metabolic switch toward enhanced oxidative phosphorylation in ccRCC, which can be related to PGC1α-mediated metabolic networks. Therefore, this current study lays the foundation for the further development of a global metabolic analysis of cancer cells in individual patients, which ultimately will have significant potential for the discovery of novel therapeutics and precision medicine in SETD2-inactivated ccRCC.
中文翻译:

SETD2 缺失会诱导肾细胞癌细胞系代谢转变为增强氧化磷酸化
SETD2 是一种组蛋白 H3 赖氨酸三甲基转移酶,经常失活并与透明细胞肾细胞癌 (ccRCC) 的复发相关。然而,SETD2 缺失对 ccRCC 代谢改变的影响仍不清楚。在本研究中,通过锌指核酸酶产生源自786-O细胞的SETD2无效同基因38E/38F克隆,并分别通过靶向代谢组学、RNA测序和生物学方法分析随后的代谢、基因组和细胞表型变化。我们的结果表明,与亲代 786-O 细胞相比,38E/38F 细胞的 MTT/Alamar 蓝水平、ATP、糖酵解/线粒体呼吸能力、柠檬酸合酶 (CS) 活性和 TCA 代谢物(如天冬氨酸、苹果酸、琥珀酸、富马酸和α-酮戊二酸。 38E/38F 细胞还利用丙酮酸以外的替代来源来生成用于 TCA 循环的乙酰辅酶 A。此外,38E/38F细胞显示出主要与线粒体代谢以及脂肪酸和葡萄糖氧化相关的基因网络紊乱,这与PGC1α、线粒体质量和细胞大小/复杂性的增加有关。我们的结果表明,SETD2 缺陷会诱导 ccRCC 中氧化磷酸化的代谢转变,这可能与 PGC1α 介导的代谢网络有关。因此,当前的研究为进一步发展个体患者癌细胞的整体代谢分析奠定了基础,最终将为发现 SETD2 失活 ccRCC 的新型疗法和精准医学带来巨大潜力。
更新日期:2018-11-28
中文翻译:

SETD2 缺失会诱导肾细胞癌细胞系代谢转变为增强氧化磷酸化
SETD2 是一种组蛋白 H3 赖氨酸三甲基转移酶,经常失活并与透明细胞肾细胞癌 (ccRCC) 的复发相关。然而,SETD2 缺失对 ccRCC 代谢改变的影响仍不清楚。在本研究中,通过锌指核酸酶产生源自786-O细胞的SETD2无效同基因38E/38F克隆,并分别通过靶向代谢组学、RNA测序和生物学方法分析随后的代谢、基因组和细胞表型变化。我们的结果表明,与亲代 786-O 细胞相比,38E/38F 细胞的 MTT/Alamar 蓝水平、ATP、糖酵解/线粒体呼吸能力、柠檬酸合酶 (CS) 活性和 TCA 代谢物(如天冬氨酸、苹果酸、琥珀酸、富马酸和α-酮戊二酸。 38E/38F 细胞还利用丙酮酸以外的替代来源来生成用于 TCA 循环的乙酰辅酶 A。此外,38E/38F细胞显示出主要与线粒体代谢以及脂肪酸和葡萄糖氧化相关的基因网络紊乱,这与PGC1α、线粒体质量和细胞大小/复杂性的增加有关。我们的结果表明,SETD2 缺陷会诱导 ccRCC 中氧化磷酸化的代谢转变,这可能与 PGC1α 介导的代谢网络有关。因此,当前的研究为进一步发展个体患者癌细胞的整体代谢分析奠定了基础,最终将为发现 SETD2 失活 ccRCC 的新型疗法和精准医学带来巨大潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号