当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mammary fibroblasts remodel fibrillar collagen microstructure in a biomimetic nanocomposite hydrogel.
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-11-07 , DOI: 10.1016/j.actbio.2018.11.010 Chun Liu 1 , Benjamin Chiang 1 , Daniela Lewin Mejia 1 , Kathryn E Luker 1 , Gary D Luker 2 , Andre Lee 3
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-11-07 , DOI: 10.1016/j.actbio.2018.11.010 Chun Liu 1 , Benjamin Chiang 1 , Daniela Lewin Mejia 1 , Kathryn E Luker 1 , Gary D Luker 2 , Andre Lee 3
Affiliation
|
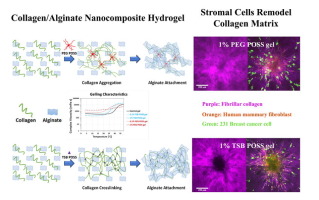
Architecture and microstructure of type I collagen fibers constitute central regulators of tumor invasion with aligned fibers providing a route for migration of stromal and cancer cells. Several different aspects of fibrillar collagen, such as stiffness, density, thickness, and pore size, may regulate migration of cancer cells, but determining effects of any one parameter requires clear decoupling of physical properties of collagen networks. The objective of this work is to develop and apply an in vitro three-dimensional (3D) tumor-extra cellular matrix (ECM) model with tunable physical parameters to define how stromal fibroblasts modulate collagen microstructure to control migration of breast cancer cells. We incorporated two different types of polyhedral oligomeric silsesquioxane (POSS) nano-molecules into a collagen/alginate matrix to induce different mechanisms of gelling. The resultant biomimetic, nanocomposite hydrogels show different collagen fibrillar microstructures while maintaining constant overall matrix stiffness, density, and porosimetry. Spheroids of human mammary fibroblasts embedded in these 3D matrices remodel the collagen network to varying extents based on differences in underlying matrix microstructures. The remodeled collagen matrix shows oriented, thicker fibrillar tracks, facilitating invasion of tumor cells. By decoupling effects of matrix stiffness and architecture, our nanocomposite hydrogels serve as robust platforms to investigate how biophysical properties of tumor environments control key processes regulating tumor progression in breast cancer and other malignancies. STATEMENT OF SIGNIFICANCE: Our manuscript demonstrates a new type of nanocomposite hydrogel with two different gelling mechanisms, produced by incorporating two types of polyhedral oligomeric silsesquioxane (POSS) nano-molecules into a collagen/alginate matrix. The resultant biomimetic hydrogels show different fibrillar collagen microstructures while maintaining constant overall matrix stiffness, density, and porosimetry. These gels allow us to uncouple effects of matrix stiffness versus architecture on migration and invasion of breast cancer cells and stromal fibroblasts. Upon embedding spheroids of human mammary fibroblasts (HMFs) and dissociated 231 breast cancer cells, we showed that HMFs remodeled the collagen network to differing extents dependent on starting matrix microstructures in each hydrogel. The remodeled collagen matrix showed aligned collagen fibers perpendicular to the surface of a spheroid with migrating HMFs following these fibers as occurs in tumors in vivo. To our knowledge, this is the first study showing significant different fibrillar collagen microstructures with constant collagen density and gel stiffness. This study establishes a new type of nanocomposite 3D hydrogels for studies of biophysical and cellular interactions in engineered tumor environments.
中文翻译:

乳腺成纤维细胞在仿生纳米复合水凝胶中重塑纤维状胶原微结构。
I型胶原纤维的结构和微结构构成了肿瘤侵袭的中央调节器,排列的纤维为基质细胞和癌细胞的迁移提供了途径。纤维状胶原的几个不同方面,例如刚度,密度,厚度和孔径,可能会调节癌细胞的迁移,但是要确定任何一个参数的作用,都需要胶原网络的物理特性清楚地分离。这项工作的目的是开发和应用具有可调节物理参数的体外三维(3D)肿瘤外细胞基质(ECM)模型,以定义基质成纤维细胞如何调节胶原微结构以控制乳腺癌细胞的迁移。我们将两种不同类型的多面体低聚倍半硅氧烷(POSS)纳米分子整合到胶原蛋白/藻酸盐基质中,以诱导不同的胶凝机制。所得的仿生纳米复合水凝胶显示出不同的胶原原纤维微结构,同时保持了恒定的整体基质刚度,密度和孔隙率。嵌入在这些3D矩阵中的人类乳腺成纤维细胞的球体会根据基础基质微结构的差异,在不同程度上重塑胶原网络。重塑的胶原蛋白基质显示定向的较粗的纤维状痕迹,促进肿瘤细胞的侵袭。通过解耦矩阵刚度和结构的影响,我们的纳米复合水凝胶可作为强大的平台,用于研究肿瘤环境的生物物理特性如何控制调节乳腺癌和其他恶性肿瘤中肿瘤进展的关键过程。意义声明:我们的手稿演示了一种具有两种不同胶凝机理的新型纳米复合水凝胶,该胶水是通过将两种类型的多面体低聚倍半硅氧烷(POSS)纳米分子掺入胶原/藻酸盐基质中制成的。所得仿生水凝胶显示出不同的原纤维胶原微结构,同时保持恒定的整体基质刚度,密度和孔隙率。这些凝胶使我们能够消除基质刚度与结构对乳腺癌细胞和基质成纤维细胞迁移和侵袭的影响。嵌入人类乳腺成纤维细胞(HMFs)的球状体并解离了231个乳腺癌细胞后,我们发现HMFs在不同程度上重塑了胶原蛋白网络,具体取决于每个水凝胶中起始基质的微结构。重塑的胶原蛋白基质显示出与球状体表面垂直的对齐的胶原蛋白纤维,并且在体内的肿瘤中会随着这些纤维的迁移而迁移HMF。据我们所知,这是第一项研究,显示了具有不变的胶原蛋白密度和凝胶刚度的明显不同的原纤维胶原蛋白微结构。这项研究建立了一种新型的纳米复合3D水凝胶,用于研究工程化肿瘤环境中的生物物理和细胞相互作用。我们表明,HMFs可以根据每个水凝胶中起始基质的微观结构不同程度地重塑胶原蛋白网络。重塑的胶原蛋白基质显示出与球状体表面垂直的对齐的胶原蛋白纤维,并且在体内的肿瘤中会随着这些纤维的迁移而迁移HMF。据我们所知,这是第一项研究,显示了具有不变的胶原蛋白密度和凝胶刚度的明显不同的原纤维胶原蛋白微结构。这项研究建立了一种新型的纳米复合3D水凝胶,用于研究工程化肿瘤环境中的生物物理和细胞相互作用。我们表明,HMFs可以根据每个水凝胶中起始基质的微观结构不同程度地重塑胶原蛋白网络。重塑的胶原蛋白基质显示出与球状体表面垂直的对齐的胶原蛋白纤维,并且在体内的肿瘤中会随着这些纤维的迁移而迁移HMF。据我们所知,这是第一项研究,显示了具有不变的胶原蛋白密度和凝胶刚度的明显不同的原纤维胶原蛋白微结构。这项研究建立了一种新型的纳米复合3D水凝胶,用于研究工程化肿瘤环境中的生物物理和细胞相互作用。这是第一项研究,显示出具有不变的胶原蛋白密度和凝胶刚度的显着不同的原纤维胶原蛋白微结构。这项研究建立了一种新型的纳米复合3D水凝胶,用于研究工程化肿瘤环境中的生物物理和细胞相互作用。这是第一项研究,显示出具有不变的胶原蛋白密度和凝胶刚度的显着不同的原纤维胶原蛋白微结构。这项研究建立了一种新型的纳米复合3D水凝胶,用于研究工程化肿瘤环境中的生物物理和细胞相互作用。
更新日期:2018-11-08
中文翻译:

乳腺成纤维细胞在仿生纳米复合水凝胶中重塑纤维状胶原微结构。
I型胶原纤维的结构和微结构构成了肿瘤侵袭的中央调节器,排列的纤维为基质细胞和癌细胞的迁移提供了途径。纤维状胶原的几个不同方面,例如刚度,密度,厚度和孔径,可能会调节癌细胞的迁移,但是要确定任何一个参数的作用,都需要胶原网络的物理特性清楚地分离。这项工作的目的是开发和应用具有可调节物理参数的体外三维(3D)肿瘤外细胞基质(ECM)模型,以定义基质成纤维细胞如何调节胶原微结构以控制乳腺癌细胞的迁移。我们将两种不同类型的多面体低聚倍半硅氧烷(POSS)纳米分子整合到胶原蛋白/藻酸盐基质中,以诱导不同的胶凝机制。所得的仿生纳米复合水凝胶显示出不同的胶原原纤维微结构,同时保持了恒定的整体基质刚度,密度和孔隙率。嵌入在这些3D矩阵中的人类乳腺成纤维细胞的球体会根据基础基质微结构的差异,在不同程度上重塑胶原网络。重塑的胶原蛋白基质显示定向的较粗的纤维状痕迹,促进肿瘤细胞的侵袭。通过解耦矩阵刚度和结构的影响,我们的纳米复合水凝胶可作为强大的平台,用于研究肿瘤环境的生物物理特性如何控制调节乳腺癌和其他恶性肿瘤中肿瘤进展的关键过程。意义声明:我们的手稿演示了一种具有两种不同胶凝机理的新型纳米复合水凝胶,该胶水是通过将两种类型的多面体低聚倍半硅氧烷(POSS)纳米分子掺入胶原/藻酸盐基质中制成的。所得仿生水凝胶显示出不同的原纤维胶原微结构,同时保持恒定的整体基质刚度,密度和孔隙率。这些凝胶使我们能够消除基质刚度与结构对乳腺癌细胞和基质成纤维细胞迁移和侵袭的影响。嵌入人类乳腺成纤维细胞(HMFs)的球状体并解离了231个乳腺癌细胞后,我们发现HMFs在不同程度上重塑了胶原蛋白网络,具体取决于每个水凝胶中起始基质的微结构。重塑的胶原蛋白基质显示出与球状体表面垂直的对齐的胶原蛋白纤维,并且在体内的肿瘤中会随着这些纤维的迁移而迁移HMF。据我们所知,这是第一项研究,显示了具有不变的胶原蛋白密度和凝胶刚度的明显不同的原纤维胶原蛋白微结构。这项研究建立了一种新型的纳米复合3D水凝胶,用于研究工程化肿瘤环境中的生物物理和细胞相互作用。我们表明,HMFs可以根据每个水凝胶中起始基质的微观结构不同程度地重塑胶原蛋白网络。重塑的胶原蛋白基质显示出与球状体表面垂直的对齐的胶原蛋白纤维,并且在体内的肿瘤中会随着这些纤维的迁移而迁移HMF。据我们所知,这是第一项研究,显示了具有不变的胶原蛋白密度和凝胶刚度的明显不同的原纤维胶原蛋白微结构。这项研究建立了一种新型的纳米复合3D水凝胶,用于研究工程化肿瘤环境中的生物物理和细胞相互作用。我们表明,HMFs可以根据每个水凝胶中起始基质的微观结构不同程度地重塑胶原蛋白网络。重塑的胶原蛋白基质显示出与球状体表面垂直的对齐的胶原蛋白纤维,并且在体内的肿瘤中会随着这些纤维的迁移而迁移HMF。据我们所知,这是第一项研究,显示了具有不变的胶原蛋白密度和凝胶刚度的明显不同的原纤维胶原蛋白微结构。这项研究建立了一种新型的纳米复合3D水凝胶,用于研究工程化肿瘤环境中的生物物理和细胞相互作用。这是第一项研究,显示出具有不变的胶原蛋白密度和凝胶刚度的显着不同的原纤维胶原蛋白微结构。这项研究建立了一种新型的纳米复合3D水凝胶,用于研究工程化肿瘤环境中的生物物理和细胞相互作用。这是第一项研究,显示出具有不变的胶原蛋白密度和凝胶刚度的显着不同的原纤维胶原蛋白微结构。这项研究建立了一种新型的纳米复合3D水凝胶,用于研究工程化肿瘤环境中的生物物理和细胞相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号