当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The High Chemofidelity of Metal-Catalyzed Hydrogen Atom Transfer
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-11-08 00:00:00 , DOI: 10.1021/acs.accounts.8b00337 Samantha A. Green 1 , Steven W. M. Crossley 1 , Jeishla L. M. Matos 1 , Suhelen Vásquez-Céspedes 1 , Sophia L. Shevick 1 , Ryan A. Shenvi 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-11-08 00:00:00 , DOI: 10.1021/acs.accounts.8b00337 Samantha A. Green 1 , Steven W. M. Crossley 1 , Jeishla L. M. Matos 1 , Suhelen Vásquez-Céspedes 1 , Sophia L. Shevick 1 , Ryan A. Shenvi 1
Affiliation
|
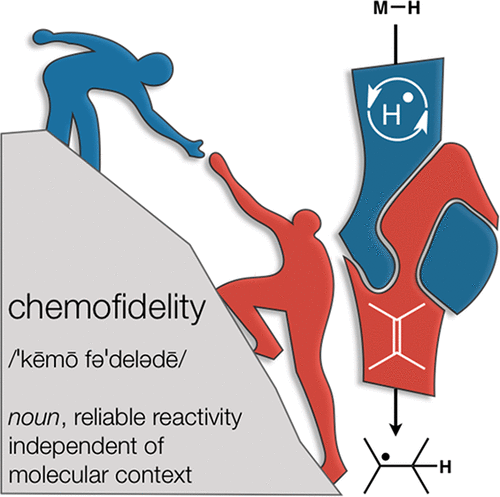
The implementation of any chemical reaction in a structurally complex setting (King, S. M. J. Org. Chem. 2014, 79, 8937) confronts structurally defined barriers: steric environment, functional group reactivity, product instability, and through-bond electronics. However, there are also practical barriers. Late-stage reactions conducted on small quantities of material are run inevitably at lower than optimal concentrations. Access to late-stage material limits extensive optimization. Impurities from past reactions can interfere, especially with catalytic reactions. Therefore, chemical reactions on which one can rely at the front lines of a complex synthesis campaign emerge from the crucible of total synthesis as robust, dependable, and widely applied. Trost conceptualized “chemoselectivity” as a reagent’s selective reaction of one functional group or reactive site in preference to others (Trost, B. M. Science 1983, 219, 245). Chemoselectivity and functional group tolerance can be evaluated quickly using robustness screens (Collins, K. D. Nat. Chem. 2013, 5, 597). A reaction may also be characterized by its “chemofidelity”, that is, its reliable reaction with a functional group in any molecular context. For example, ketone reduction by an electride (dissolving metal conditions) exhibits high chemofidelity but low chemoselectivity: it usually works, but many other functional groups are reduced at similar rates. Conversely, alkene coordination chemistry effected by π Lewis acids can exhibit high chemoselectivity (Trost, B. M. Science 1983, 219, 245) but low chemofidelity: it can be highly selective for alkenes but sensitive to the substitution pattern (Larionov, E. Chem. Commun. 2014, 50, 9816). In contrast, alkenes undergo reliable, robust, and diverse hydrogen atom transfer reactions from metal hydrides to generate carbon-centered radicals. Although there are many potential applications of this chemistry, its functional group tolerance, high rates, and ease of execution have led to its rapid deployment in complex synthesis campaigns. Its success derives from high chemofidelity, that is, its dependable reactivity in many molecular environments and with many alkene substitution patterns. Metal hydride H atom transfer (MHAT) reactions convert diverse, simple building blocks to more stereochemically and functionally dense products (Crossley, S. W. M. Chem. Rev. 2016, 116, 8912). When hydrogen is returned to the metal, MHAT can be considered the radical equivalent of Brønsted acid catalysis—itself a broad reactivity paradigm. This Account summarizes our group’s contributions to method development, reagent discovery, and mechanistic interrogation. Our earliest contribution to this area—a stepwise hydrogenation with high chemoselectivity and high chemofidelity—has found application to many problems. More recently, we reported the first examples of dual-catalytic cross-couplings that rely on the merger of MHAT cycles and nickel catalysis. With time, we anticipate that MHAT will become a staple of chemical synthesis.
中文翻译:

金属催化氢原子转移的高化学保真度
在结构复杂的环境中实施任何化学反应(金,SM J.Org。化学 2014, 79,8937)面对结构上定义的障碍:空间环境中,官能团的反应性,产品不稳定,并通过键合电子元件。但是,也存在实际障碍。在少量物料上进行的后期反应不可避免地在低于最佳浓度的条件下进行。后期材料的使用限制了广泛的优化。过去反应中的杂质可能会干扰,特别是催化反应。因此,可以依靠一个复杂的合成运动的最前线的化学反应从全合成的坩埚中出现,它是可靠,可靠和广泛应用的。Trost将“化学选择性”概念化为一个功能团或反应位点优先于其他功能团或反应位点的试剂选择性反应(特洛斯特(BM) 科学 1983, 219,245)。化学选择性和官能团耐受性可使用健壮性屏幕快速评估(柯林斯(KD) 纳特 化学 2013, 5,597)。反应还可以通过其“化学异构性”来表征,即在任何分子环境中与官能团的可靠反应。例如,通过电子化合物(溶解的金属条件)使酮还原显示出较高的化学纤维度,但化学选择性低:通常起作用,但是许多其他官能团的还原速率相似。相反,受π路易斯酸影响的烯烃配位化学可表现出较高的化学选择性(特洛斯特(BM) 科学 1983, 219,245),但低chemofidelity:它可以是用于烯烃高选择性,但敏感的取代模式(拉里奥诺夫(E. Larionov) 化学 公社 2014, 50,9816)。相反,烯烃从金属氢化物进行可靠,稳定且多样的氢原子转移反应,从而生成以碳为中心的自由基。尽管这种化学有许多潜在的应用,但其功能基团的耐受性,高速率和易于实施已使其在复杂的合成反应中迅速部署。它的成功源于高化学感应度,也就是说,它在许多分子环境中和许多烯烃取代方式下均具有可靠的反应性。金属氢化物H原子转移(MHAT)反应可将各种简单的结构单元转化为更立体化学和功能密集的产物(SWM克罗斯利 化学 修订版 2016, 116,8912)。当氢返回到金属中时,MHAT可以看作是布朗斯台德酸催化的自由基等同物,它本身就是一种广泛的反应性范式。该帐户总结了我们小组对方法开发,试剂发现和机械询问的贡献。我们对此领域的最早贡献-具有高化学选择性和高化学纤维度的逐步加氢-已发现可用于许多问题。最近,我们报道了依赖于MHAT循环和镍催化作用的双重催化交叉偶联的第一个例子。随着时间的流逝,我们预计MHAT将成为化学合成的主要内容。
更新日期:2018-11-08
中文翻译:

金属催化氢原子转移的高化学保真度
在结构复杂的环境中实施任何化学反应(











































 京公网安备 11010802027423号
京公网安备 11010802027423号