当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Factors Affecting Hydrogen Atom Transfer Reactivity of Metal–Oxo Porphyrinoid Complexes
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-11-07 00:00:00 , DOI: 10.1021/acs.accounts.8b00414 Jireh Joy D. Sacramento 1 , David P. Goldberg 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-11-07 00:00:00 , DOI: 10.1021/acs.accounts.8b00414 Jireh Joy D. Sacramento 1 , David P. Goldberg 1
Affiliation
|
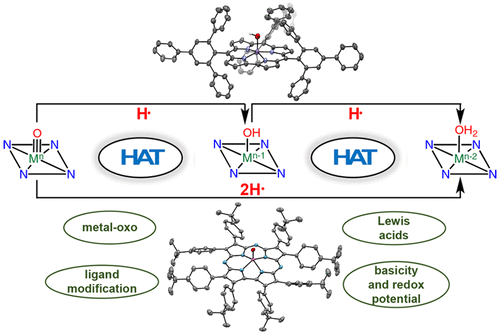
There has been considerable interest in hydrogen atom transfer (HAT) reactions mediated by metal/oxygen species because of their central role in metalloenzyme function as well as synthetic catalysts. This Account focuses on our progress in synthesizing high-valent metal–oxo and metal–hydroxo porphyrinoid complexes and determining their reactivities in a range of HAT processes. For these studies we have utilized corrolazine and corrole ligands, which are a ring-contracted subclass of porphyrinoid compounds designed to stabilize high-valent metal complexes. The high-valent manganese complex MnV(O)(TBP8Cz) (TBP8Cz = octakis(4-tert-butylphenyl)corrolazine3−) provided an early example of a well-characterized low-potential oxidant that can still be effective at abstracting H atoms from certain C–H/O–H bonds. Approximating the thermodynamics of the HAT reactivity of the MnV(O) complex and related species with the help of a square scheme approach, in which HAT can be formally separated into proton (pKa) and electron transfers (E°), indicates that affinity for the proton (i.e., the basicity) is a key factor in promoting HAT. Anionic axial ligands have a profound influence on the HAT reactivity of MnV(O)(TBP8Cz), supporting the conclusion that basicity is a critical parameter in determining the reactivity. The influence of Lewis acids on MnV(O)(TBP8Cz) was examined, and it was shown that both the electronic structure and reactivity toward HAT were significantly altered. High-valent Cr(O), Re(O), and Fe(O) corrolazines were prepared, and a range of HAT reactions were studied with these complexes. The chromium and manganese complexes form a rare pair of structurally characterized CrV(O) and MnV(O) species in identical ligand environments, allowing for a direct comparison of their HAT reactivities. Although the CrV(O) species was the better oxidant as measured by redox potentials, the MnV(O) species was significantly more reactive in HAT oxidations, pointing again to basicity as a key determinant of HAT reactivity. The iron complex, FeIV(O)(TBP8Cz+•), is an analogue of the heme enzyme Compound I intermediate, and was found to be mildly reactive toward H atom abstraction from C–H bonds. In contrast, ReV(O)(TBP8Cz) was inert toward HAT, although one-electron oxidation to ReV(O)(TBP8Cz+•) led to some interesting reactivity mediated by the π-radical-cation ligand alone. Other ligand modifications, including peripheral substitution as well as novel alkylation of the meso position on the Cz core, were examined for their influence on HAT. A highly sterically encumbered corrole, tris(2,4,6-triphenylphenyl)corrole (ttppc), was employed for the isolation and structural characterization of the first MnIV(OH) complex in a porphyrinoid environment, MnIV(OH)(ttppc). This complex was highly reactive in HAT with O–H substrates and was found to be much more reactive than its higher-oxidation-state counterpart MnV(O)(ttppc), providing important mechanistic insights. These studies provided fundamental knowledge on the relationship between structure and function in high-valent M(O) and M(OH) models of heme enzyme reactivity.
中文翻译:

影响金属-氧卟啉配合物氢原子转移反应性的因素
由于金属/氧物种在金属酶功能以及合成催化剂中的核心作用,人们对由金属/氧物种介导的氢原子转移(HAT)反应引起了极大的兴趣。该帐户重点介绍了我们在合成高价金属-氧和金属-羟基卟啉类配合物并确定其在一系列HAT工艺中的反应性方面的进展。在这些研究中,我们利用了corrolazine和corrole配体,它们是设计成稳定高价金属络合物的卟啉类化合物的环状缩合亚类。高价锰配合物Mn V(O)(TBP 8 Cz)(TBP 8 Cz =八(4-叔丁基苯基)Corrolazine 3-)提供了一个表征良好的低电位氧化剂的早期实例,该氧化剂仍然可以有效地从某些C–H / O–H键中提取H原子。借助于方方案方法,近似估算了Mn V(O)配合物和相关物种的HAT反应性的热力学,其中HAT可以被正式分离为质子(p K a)和电子传递(E °),与质子的亲和力(即碱性)是促进HAT的关键因素。阴离子轴向配体对Mn V(O)(TBP 8 Cz)的HAT反应性具有深远的影响,支持以下结论:碱度是决定反应性的关键参数。路易斯酸对锰的影响检测了V(O)(TBP 8 Cz),结果表明,其电子结构和对HAT的反应性均发生了显着变化。制备了高价的Cr(O),Re(O)和Fe(O)Corrolazines,并用这些配合物研究了一系列HAT反应。铬和锰的络合物在相同的配体环境中形成了稀有的结构特征化的Cr V(O)和Mn V(O)对,从而可以直接比较它们的HAT反应性。尽管通过氧化还原电势测得Cr V(O)物种是更好的氧化剂,但Mn V(O)物种在HAT氧化中的反应性明显更高,再次指出碱性是HAT反应性的关键决定因素。铁络合物Fe IV(O)(TBP 8 Cz +•)是血红素酶化合物I中间体的类似物,被发现对C–H键中的H原子有轻度反应。相反,尽管单电子氧化为Re V(O)(TBP 8 Cz +•,Re V(O)(TBP 8 Cz)对HAT是惰性的)导致仅由π-自由基阳离子配体介导的一些有趣的反应性。研究了其他配体修饰,包括外围取代以及Cz核上内消旋位置的新型烷基化,对它们对HAT的影响。使用高度立体阻碍的三(2,4,6-三苯基苯基)甲酸酯(ttppc)来分离和表征卟啉类环境Mn IV(OH)(ttppc)中的第一个Mn IV(OH)配合物)。该配合物在具有O–H底物的HAT中具有高反应性,并且发现其反应性比其高氧化态对应物Mn V高得多。(O)(ttppc),提供重要的机械见解。这些研究为血红素酶反应性的高价M(O)和M(OH)模型中的结构与功能之间的关系提供了基础知识。
更新日期:2018-11-07
中文翻译:

影响金属-氧卟啉配合物氢原子转移反应性的因素
由于金属/氧物种在金属酶功能以及合成催化剂中的核心作用,人们对由金属/氧物种介导的氢原子转移(HAT)反应引起了极大的兴趣。该帐户重点介绍了我们在合成高价金属-氧和金属-羟基卟啉类配合物并确定其在一系列HAT工艺中的反应性方面的进展。在这些研究中,我们利用了corrolazine和corrole配体,它们是设计成稳定高价金属络合物的卟啉类化合物的环状缩合亚类。高价锰配合物Mn V(O)(TBP 8 Cz)(TBP 8 Cz =八(4-叔丁基苯基)Corrolazine 3-)提供了一个表征良好的低电位氧化剂的早期实例,该氧化剂仍然可以有效地从某些C–H / O–H键中提取H原子。借助于方方案方法,近似估算了Mn V(O)配合物和相关物种的HAT反应性的热力学,其中HAT可以被正式分离为质子(p K a)和电子传递(E °),与质子的亲和力(即碱性)是促进HAT的关键因素。阴离子轴向配体对Mn V(O)(TBP 8 Cz)的HAT反应性具有深远的影响,支持以下结论:碱度是决定反应性的关键参数。路易斯酸对锰的影响检测了V(O)(TBP 8 Cz),结果表明,其电子结构和对HAT的反应性均发生了显着变化。制备了高价的Cr(O),Re(O)和Fe(O)Corrolazines,并用这些配合物研究了一系列HAT反应。铬和锰的络合物在相同的配体环境中形成了稀有的结构特征化的Cr V(O)和Mn V(O)对,从而可以直接比较它们的HAT反应性。尽管通过氧化还原电势测得Cr V(O)物种是更好的氧化剂,但Mn V(O)物种在HAT氧化中的反应性明显更高,再次指出碱性是HAT反应性的关键决定因素。铁络合物Fe IV(O)(TBP 8 Cz +•)是血红素酶化合物I中间体的类似物,被发现对C–H键中的H原子有轻度反应。相反,尽管单电子氧化为Re V(O)(TBP 8 Cz +•,Re V(O)(TBP 8 Cz)对HAT是惰性的)导致仅由π-自由基阳离子配体介导的一些有趣的反应性。研究了其他配体修饰,包括外围取代以及Cz核上内消旋位置的新型烷基化,对它们对HAT的影响。使用高度立体阻碍的三(2,4,6-三苯基苯基)甲酸酯(ttppc)来分离和表征卟啉类环境Mn IV(OH)(ttppc)中的第一个Mn IV(OH)配合物)。该配合物在具有O–H底物的HAT中具有高反应性,并且发现其反应性比其高氧化态对应物Mn V高得多。(O)(ttppc),提供重要的机械见解。这些研究为血红素酶反应性的高价M(O)和M(OH)模型中的结构与功能之间的关系提供了基础知识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号