当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactions of Folded Molecules in Water
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-11-06 00:00:00 , DOI: 10.1021/acs.accounts.8b00269 Yang Yu 1 , Julius Rebek 1, 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-11-06 00:00:00 , DOI: 10.1021/acs.accounts.8b00269 Yang Yu 1 , Julius Rebek 1, 2
Affiliation
|
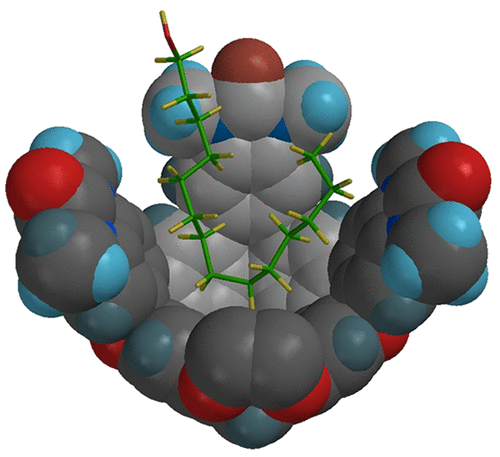
The chemistry of confined molecules is a relatively new undertaking, and this Account describes the effects of certain host container compounds on the behavior of molecules held as guests within. The containers are known as cavitands, which have one open end that allows small molecules to go in and out. The containers are amphiphilic: they feature aromatic surfaces that create a hydrophobic space inside but their peripheries are polar and permit solubility in water. The tension between the inner space of the cavitand and the outer space of the medium is experienced by the guest molecules. Two kinds of cavitand, a cylindrical and a cone-like methylated cavitand, are presented here, and they bind guests in somewhat different depths. The cylindrical cavitand typically has its aromatic panels closer to the guests and when a suitable guest is inside, two cylindrical cavitands can form a capsule through hydrogen bonding between their rims. Halogen bonding may also occur between the aromatic faces of the cavitands and the “sigma hole” of appropriate halides. Long-chain organic compounds of suitable size form host/guest complexes through hydrophobic forces on brief sonication with both cavitands in water. The container’s shape acts on flexible guests and deforms them in order to fill the space properly. NMR spectroscopy reveals that many long-chain guests assume U- or J-shaped conformations within the cavitands. The J-shaped conformations are dynamic and undergo “yo-yo” like motions in the cavitand. An inevitable consequence of a folded chain is that its ends are closer together. Accordingly, these guests are prone to cyclization processes.
中文翻译:

折叠分子在水中的反应
受限分子的化学性质是一个相对较新的任务,该帐户描述了某些宿主容器化合物对作为客体的分子行为的影响。这些容器被称为腔体,其具有一个开放端,允许小分子进出。这些容器是两亲性的:它们具有芳香的表面,可以在内部形成疏水性空间,但是它们的周边是极性的,可以在水中溶解。空穴分子的内部空间和介质的外部空间之间的张力是由客体分子承受的。这里介绍了两种空泡,分别是圆柱状和圆锥形的甲基化空泡,它们以不同的深度束缚客人。圆柱型腔体通常具有更靠近宾客的芳香板,并且当合适的宾客进入室内时,两个圆柱形的空洞可以通过其边缘之间的氢键形成胶囊。空穴的芳香面和适当的卤化物的“σ孔”之间也可能发生卤素键合。适当大小的长链有机化合物通过在水中与两种空泡石进行短暂超声处理时的疏水作用力形成宿主/客体复合物。容器的形状作用在可弯曲的客人身上并使之变形,以正确填充空间。NMR光谱显示,许多长链客体在空洞中呈U形或J形构象。J形构象是动态的,并且像cavitand中的运动一样经历“溜溜球”运动。折叠链的必然结果是 空穴的芳香面和适当的卤化物的“σ孔”之间也可能发生卤素键合。适当大小的长链有机化合物通过在水中与两种空泡石进行短暂超声处理时的疏水作用力形成宿主/客体复合物。容器的形状作用在可弯曲的客人身上并使之变形,以正确填充空间。NMR光谱显示,许多长链客体在空洞中呈U形或J形构象。J形构象是动态的,并且像cavitand中的运动一样经历“溜溜球”运动。折叠链的必然结果是 空穴的芳香面和适当的卤化物的“σ孔”之间也可能发生卤素键合。适当大小的长链有机化合物通过在水中与两种空泡石进行短暂超声处理时的疏水作用力形成宿主/客体复合物。容器的形状作用在可弯曲的客人身上并使之变形,以正确填充空间。NMR光谱显示,许多长链客体在空洞中呈U形或J形构象。J形构象是动态的,并且像cavitand中的运动一样经历“溜溜球”运动。折叠链的必然结果是 容器的形状作用在可弯曲的客人身上并使之变形,以正确填充空间。NMR光谱显示,许多长链客体在空洞中呈U形或J形构象。J形构象是动态的,并且像cavitand中的运动一样经历“溜溜球”运动。折叠链的必然结果是 容器的形状作用在可弯曲的客人身上并使之变形,以正确填充空间。NMR光谱显示,许多长链客体在空洞中呈U形或J形构象。J形构象是动态的,并且像cavitand中的运动一样经历“溜溜球”运动。折叠链的必然结果是它的两端靠得更近。因此,这些来宾倾向于环化过程。
更新日期:2018-11-06
中文翻译:

折叠分子在水中的反应
受限分子的化学性质是一个相对较新的任务,该帐户描述了某些宿主容器化合物对作为客体的分子行为的影响。这些容器被称为腔体,其具有一个开放端,允许小分子进出。这些容器是两亲性的:它们具有芳香的表面,可以在内部形成疏水性空间,但是它们的周边是极性的,可以在水中溶解。空穴分子的内部空间和介质的外部空间之间的张力是由客体分子承受的。这里介绍了两种空泡,分别是圆柱状和圆锥形的甲基化空泡,它们以不同的深度束缚客人。圆柱型腔体通常具有更靠近宾客的芳香板,并且当合适的宾客进入室内时,两个圆柱形的空洞可以通过其边缘之间的氢键形成胶囊。空穴的芳香面和适当的卤化物的“σ孔”之间也可能发生卤素键合。适当大小的长链有机化合物通过在水中与两种空泡石进行短暂超声处理时的疏水作用力形成宿主/客体复合物。容器的形状作用在可弯曲的客人身上并使之变形,以正确填充空间。NMR光谱显示,许多长链客体在空洞中呈U形或J形构象。J形构象是动态的,并且像cavitand中的运动一样经历“溜溜球”运动。折叠链的必然结果是 空穴的芳香面和适当的卤化物的“σ孔”之间也可能发生卤素键合。适当大小的长链有机化合物通过在水中与两种空泡石进行短暂超声处理时的疏水作用力形成宿主/客体复合物。容器的形状作用在可弯曲的客人身上并使之变形,以正确填充空间。NMR光谱显示,许多长链客体在空洞中呈U形或J形构象。J形构象是动态的,并且像cavitand中的运动一样经历“溜溜球”运动。折叠链的必然结果是 空穴的芳香面和适当的卤化物的“σ孔”之间也可能发生卤素键合。适当大小的长链有机化合物通过在水中与两种空泡石进行短暂超声处理时的疏水作用力形成宿主/客体复合物。容器的形状作用在可弯曲的客人身上并使之变形,以正确填充空间。NMR光谱显示,许多长链客体在空洞中呈U形或J形构象。J形构象是动态的,并且像cavitand中的运动一样经历“溜溜球”运动。折叠链的必然结果是 容器的形状作用在可弯曲的客人身上并使之变形,以正确填充空间。NMR光谱显示,许多长链客体在空洞中呈U形或J形构象。J形构象是动态的,并且像cavitand中的运动一样经历“溜溜球”运动。折叠链的必然结果是 容器的形状作用在可弯曲的客人身上并使之变形,以正确填充空间。NMR光谱显示,许多长链客体在空洞中呈U形或J形构象。J形构象是动态的,并且像cavitand中的运动一样经历“溜溜球”运动。折叠链的必然结果是它的两端靠得更近。因此,这些来宾倾向于环化过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号