当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Jones–Ray Effect Is Not Caused by Surface-Active Impurities
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-11-06 00:00:00 , DOI: 10.1021/acs.jpclett.8b02957 Halil I. Okur 1 , Chad I. Drexler , Eric Tyrode 1, 2 , Paul S. Cremer , Sylvie Roke 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-11-06 00:00:00 , DOI: 10.1021/acs.jpclett.8b02957 Halil I. Okur 1 , Chad I. Drexler , Eric Tyrode 1, 2 , Paul S. Cremer , Sylvie Roke 1
Affiliation
|
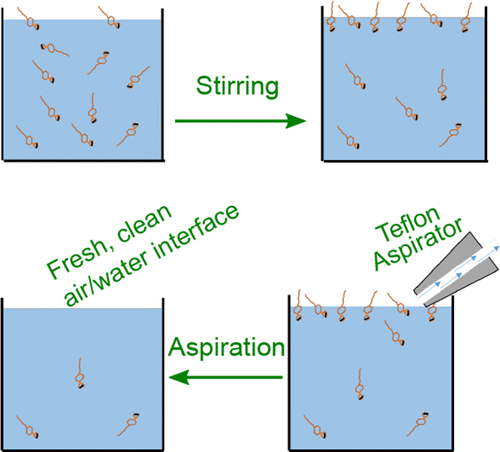
Pure aqueous electrolyte solutions display a minimum in surface tension at concentrations of 2 ± 1 mM. This effect has been a source of controversy since it was first reported by Jones and Ray in the 1930s. The Jones–Ray effect has frequently been dismissed as an artifact linked to the presence of surface-active impurities. Herein we systematically consider the effect of surface-active impurities by purposely adding nanomolar concentrations of surfactants to dilute electrolyte solutions. Trace amounts of surfactant are indeed found to decrease the surface tension and influence the surface chemistry. However, surfactants can be removed by repeated aspiration and stirring cycles, which eventually deplete the surfactant from solution, creating a pristine surface. Upon following this cleaning procedure, a reduction in the surface tension by millimolar concentrations of salt is still observed. Consequently, we demonstrate that the Jones–Ray effect is not caused by surface-active impurities.
中文翻译:

琼斯射线效应不是由表面活性杂质引起的
纯电解质水溶液在2±1 mM的浓度下显示出最小的表面张力。自从1930年代Jones和Ray首次报道以来,这种效应一直是引起争议的根源。琼斯射线效应经常被认为是与表面活性杂质的存在有关的伪影。本文中,我们通过有目的地添加纳摩尔浓度的表面活性剂来稀释电解质溶液,系统地考虑了表面活性杂质的影响。确实发现了痕量的表面活性剂会降低表面张力并影响表面化学性质。然而,表面活性剂可以通过重复的抽吸和搅拌循环除去,这最终使溶液中的表面活性剂消耗ple尽,形成了原始的表面。遵循此清洁程序后,仍然观察到表面张力降低了毫摩尔浓度的盐。因此,我们证明了琼斯射线效应不是由表面活性杂质引起的。
更新日期:2018-11-06
中文翻译:

琼斯射线效应不是由表面活性杂质引起的
纯电解质水溶液在2±1 mM的浓度下显示出最小的表面张力。自从1930年代Jones和Ray首次报道以来,这种效应一直是引起争议的根源。琼斯射线效应经常被认为是与表面活性杂质的存在有关的伪影。本文中,我们通过有目的地添加纳摩尔浓度的表面活性剂来稀释电解质溶液,系统地考虑了表面活性杂质的影响。确实发现了痕量的表面活性剂会降低表面张力并影响表面化学性质。然而,表面活性剂可以通过重复的抽吸和搅拌循环除去,这最终使溶液中的表面活性剂消耗ple尽,形成了原始的表面。遵循此清洁程序后,仍然观察到表面张力降低了毫摩尔浓度的盐。因此,我们证明了琼斯射线效应不是由表面活性杂质引起的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号