Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-11-03 , DOI: 10.1016/j.cplett.2018.10.085 Rafael F. Dias , Cleidineia C. da Costa , Taise M. Manhabosco , Alan B. de Oliveira , Matheus J.S. Matos , Jaqueline S. Soares , Ronaldo J.C. Batista
|
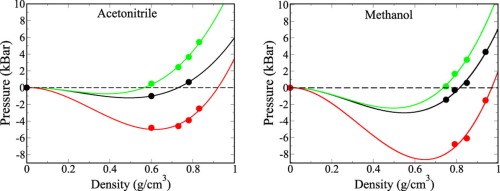
We employed PBE and BLYP semi-local functionals and the van der Waals density functional of Dion et al. [Phys. Rev. Lett. 92, 246401 (2004)] (vdW-DF) to investigate structural properties of liquid acetonitrile and methanol. Among those functionals the vdW-DF is the only one that correctly predicts energy minima in inter-molecular interactions between acetonitrile molecules. We found that van der Waals interactions have a negligible effect on H-bonds in methanol chains. However, it significantly increases chain packing resulting in a more dense liquid in comparison to the other two functionals. The overall trend is that the vdW-DF tends to overestimate density and bulk modulus, meanwhile the semi-local functionals tend to underestimate density. Thus, van der Waals interactions play an important role in the properties of liquids in which much stronger dipole-dipole interactions are present.
中文翻译:

和乙腈的分子动力学模拟:范德华相互作用的影响
我们采用了DBE等人的PBE和BLYP半局部功能以及van der Waals密度功能。[物理 莱特牧师 92,246401(2004)](vdW-DF)研究液体乙腈和甲醇的结构性质。在这些功能中,vdW-DF是唯一能够正确预测乙腈分子之间的分子间相互作用中的能量最小值的功能。我们发现范德华相互作用对甲醇链中的氢键影响可忽略不计。但是,与其他两种功能相比,它显着增加了链的堆积,从而导致液体密度更高。总体趋势是,vdW-DF倾向于高估密度和体积模量,而半局部功能倾向于低估密度。因此,









































 京公网安备 11010802027423号
京公网安备 11010802027423号