当前位置:
X-MOL 学术
›
Food Hydrocoll.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combined spectroscopic, molecular docking and quantum mechanics study of β-casein and ferulic acid interactions following UHT-like treatment
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2019-04-01 , DOI: 10.1016/j.foodhyd.2018.10.055 Lloyd Condict , Jasmeet Kaur , Andrew Hung , John Ashton , Stefan Kasapis
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2019-04-01 , DOI: 10.1016/j.foodhyd.2018.10.055 Lloyd Condict , Jasmeet Kaur , Andrew Hung , John Ashton , Stefan Kasapis
|
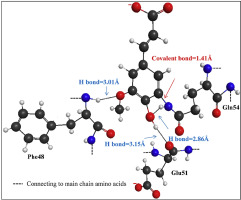
Abstract Temperature induced interactions between β-casein and ferulic acid were investigated under ultra-high temperature (UHT) conditions (140 °C) utilising a variety of spectroscopic methods. Fluorescence measurements indicate that structural alterations to the protein have occurred; the resulting red shift in tryptophan peak fluorescence with increasing concentrations of ferulic acid indicates that the single tryptophan residue has become more exposed to the polar environment. This evidence is further supported by Fourier transform infrared (FTIR) and circular dichroism (CD) measurements showing an increase in irregular and intermediary structural components upon addition of ferulic acid, as well as UV-vis measurements that record an increase in absorption. High performance liquid chromatography (HPLC) work strongly argues that the ferulic acid is bound covalently to the protein post heat treatment. This outcome is in agreement with molecular docking simulations and quantum mechanics calculations that indicate the formation of a covalent bond between ferulic acid and glutamine54 residue of β-casein to create a new adduct.
中文翻译:

UHT 样处理后β-酪蛋白和阿魏酸相互作用的组合光谱、分子对接和量子力学研究
摘要 利用各种光谱方法在超高温 (UHT) 条件 (140 °C) 下研究了 β-酪蛋白和阿魏酸之间的温度诱导相互作用。荧光测量表明蛋白质发生了结构改变;随着阿魏酸浓度的增加,色氨酸峰值荧光产生的红移表明单个色氨酸残留物更多地暴露于极性环境中。傅里叶变换红外 (FTIR) 和圆二色性 (CD) 测量结果进一步支持了这一证据,这些测量表明添加阿魏酸后不规则和中间结构成分增加,以及记录吸收增加的 UV-vis 测量结果。高效液相色谱 (HPLC) 的工作强烈主张阿魏酸在热处理后与蛋白质共价结合。这一结果与分子对接模拟和量子力学计算一致,表明阿魏酸和 β-酪蛋白的谷氨酰胺 54 残基之间形成共价键以产生新的加合物。
更新日期:2019-04-01
中文翻译:

UHT 样处理后β-酪蛋白和阿魏酸相互作用的组合光谱、分子对接和量子力学研究
摘要 利用各种光谱方法在超高温 (UHT) 条件 (140 °C) 下研究了 β-酪蛋白和阿魏酸之间的温度诱导相互作用。荧光测量表明蛋白质发生了结构改变;随着阿魏酸浓度的增加,色氨酸峰值荧光产生的红移表明单个色氨酸残留物更多地暴露于极性环境中。傅里叶变换红外 (FTIR) 和圆二色性 (CD) 测量结果进一步支持了这一证据,这些测量表明添加阿魏酸后不规则和中间结构成分增加,以及记录吸收增加的 UV-vis 测量结果。高效液相色谱 (HPLC) 的工作强烈主张阿魏酸在热处理后与蛋白质共价结合。这一结果与分子对接模拟和量子力学计算一致,表明阿魏酸和 β-酪蛋白的谷氨酰胺 54 残基之间形成共价键以产生新的加合物。









































 京公网安备 11010802027423号
京公网安备 11010802027423号